Moeassar A. Abdullah 1*; Asmaa H. Allawi 2; Taha S. Ahmed 1
1, Horticulture and Landscape Engineering Department, College of Agriculture, University of Tikrit, Tikrit, Iraq
2, Soil Science and Water Resources Department, College of Agriculture, University of Wasit, Wasit, Iraq
E-mail:
Ma.alabedy2017@tu.edu.iq
Received: 06/09/2025
Acceptance: 23/11/2025
Available Online: 24/11/2025
Published: 01/01/2026

Manuscript link
http://dx.doi.org/10.30493/DAS.2025.012411
Abstract
Mineral nutrition is one of the most effective means of improving the quantitative and qualitative characteristics of onion production systems. In semi-arid regions where alkaline soils are the most widespread type, iron deficiency chlorosis and potassium insufficiency severely limit onion yield. This experiment assessed the interactive effects of chelated iron foliar application and potassium sulfate soil application on yield parameters and the accumulation of bioactive compounds in onions under field conditions. A two-factor factorial randomized complete block design was employed, with three concentrations of chelated iron foliar spray (0, 2.25, and 4.5 g/L) and three rates of potassium sulfate (0, 150, and 300 kg K₂SO₄/ha). Standard methods were used to analyze bulb characteristics and bioactive compound content. Maximal improvements were observed with the highest treatment combination (F3K3: 4.5 g/L Fe-EDTHA + 300 kg/ha K₂SO₄), which increased fresh and dry bulb weights by 80.9% and 102% compared to control, respectively. A notable increase in phenols, flavonoids, glycosides, and terpenoids content was observed under the F3K3 treatment compared to the control. The synergistic effects of Fe and K were most pronounced in fresh and dry bulb weights. Therefore, the application of chelated iron and potassium sulfate proved to be an effective strategy for enhancing both the yield and nutritional value of onions under alkaline soil cultivation conditions.
Keywords: Onion, Chelated iron, Potassium, Foliar, Yield, Bioactive
Introduction
Onion (Allium cepa L.) is a highly profitable horticultural crop, with an annual global production of over 104.5 million tons [1]. Onion is widely used in different cuisines and is considered a key element in the human diet. Additionally, this crop has received tremendous interest due to its remarkable properties, including abundant bioactive compounds such as organosulfur compounds, phenolic acids, flavonoids, and saponins, and their significant antioxidant, anti-inflammatory, antibacterial, and cardioprotective effects [2][3]. Agricultural practices, particularly mineral nutrition management, have been shown to have immense implications for the synthesis and accumulation of these health-promoting compounds [4].
Iron deficiency is one of the most common micronutrient constraints in crop production systems, especially for vegetables cultivated in alkaline and calcareous soils [5]. Iron deficiency in onion production results in interveinal chlorosis, diminished photosynthetic capacity, and poor bulb formation [6]. In this context, the use of non-chelated iron mineral fertilizers is inefficient, since rapid precipitation and fixation are common in alkaline soils. These phenomena prompt the use of other iron fertilization methods, including chelated iron formulations [7].
Potassium is the most abundant inorganic cation in plant tissues. It is involved in the basic regulation of osmosis, activation of enzymes, protein production, and carbohydrate metabolism [8]. During onion production, optimal potassium nutrition is necessary for normal bulb development, maintenance of storage quality, and resistance to physiological disorders [9]. Potassium availability has been found to greatly affect the biosynthesis of secondary metabolites in Allium species [10].
Nutrient synergism in plant nutrition has gained increasing attention in recent years, as the combined effects of some nutrients are more influential than the sum of their individual effects [11]. Potassium ensures cellular integrity and enzyme performance, and iron facilitates electron transport mechanisms; collectively, these two elements have crucial synergistic interactions in plant metabolism [12]. Onion yield and bioactive compound production may be affected by chelated iron and potassium, but this interaction has not been thoroughly studied.
In contrast to inorganic sources of iron, chelated iron formulations display advantages that include enhanced plant uptake and nutrient retention despite alkaline soil conditions [13]. Foliar application of chelated micronutrients helps avoid soil-based limitations and provides expedient correction of deficiencies [14]. Consequently, an optimized combination of foliar chelated iron and potassium fertilization can improve onion production in terms of both yield and quality indicators under alkaline cultivation conditions. Therefore, this study was designed and conducted to: (1) determine the separate and combined effects of foliar application of chelated iron and potassium fertilization on growth and yield characteristics in onion; (2) evaluate how these treatments influence bioactive compound accumulation in onion bulbs; (3) identify the most effective treatment combinations to enhance yield and quality; and (4) develop evidence-based guidelines for integrated nutrient application in onion production systems.
Materials and Methods
Experimental location and environmental conditions
The field trial was carried out at Research Station of the College of Agriculture, University of Tikrit, Iraq (35 40 N, 43 41 E, with an altitude of 140 m above sea level) during the growing season 2017-2018. The climate at the experimental site is semi-arid continental with hot and dry summer and mild winter. The soil analysis showed alkaline conditions (7.8-8.2 pH) with moderate organic matter content (1.8-2.2%) and sufficient phosphorus availability.
Experimental design and treatments
The experiment employed a two-factorial design arranged in a Randomized Complete Block Design (RCBD) with three replicates. The experimental factors consisted of:
Factor A – Chelated Iron Foliar Application:
- F1: 0 g/L (control – distilled water spray)
- F2: 2.25 g/L chelated iron (Fe-EDTA)
- F3: 4.5 g/L chelated iron (Fe-EDTHA)
Factor B – Potassium Sulfate Application:
- K1: 0 kg K₂SO₄/ha (control)
- K2: 150 kg K₂SO₄/ha (equivalent to 72 kg K₂O/ha)
- K3: 300 kg K₂SO₄/ha (equivalent to 144 kg K₂O/ha)
The factorial combination resulted in nine treatment combinations (F1K1, F1K2, F1K3, F2K1, F2K2, F2K3, F3K1, F3K2, F3K3), each replicated three times, totaling 27 experimental units.
Field operations and crop management
The plots (4 m2) were designed to contain four planting rows with 25 cm inter-row and intra-row distance between plants and a total of 64 plants in a plot. Planting of well-established onion (Allium cepa L., cv. Texas Early Grano) transplants was done on November 15, 2017. Chelated iron was added to the solution as either Fe-EDTA (6% Fe) or Fe-EDTHA (6% Fe).
The foliar sprays were conducted once every 15 days, between 6:00 and 8:00 AM beginning three weeks after transplantation until the bulb development and a total of ten foliar sprays was given throughout the crop cycle of 150 days. Homogenous coverage and reduced drift losses were achieved by the use of hand-held sprayers with fine-mist nozzles.
K2SO4 (48% K2O + 18% S) was used as a source of potassium and was provided at three equal doses: at the time of transplanting, 45 days after transplanting (vegetative stage), and 90 days after transplanting (bulb initiation stage). The doses were added manually on the soil around the root zone after which they were irrigated instantly to improve solubility and nutrient uptake.
A drip (trickle) irrigation system was used ensuring that soil moisture did not exceed 70-80% field capacity depending on the water demand of the crops. Weed control, pest surveillance, and disease prevention were applied consistently in all treatments to reduce the variability presented by the experiment.
Data collection and sampling procedures
The onion bulbs were harvested at physiological maturity (approximately 150 days after transplanting), when 50% of plant leaves reached senescence. To avoid edge effects, five plants were randomly picked for comprehensive measurements from the central area of each plot, eliminating border plants. All analytical measurements were repeated in triplicates.
Bulb characteristics
Bulb weight (g): fresh weight of each individual bulb was measured using a sensitive analytical balance (deviation 0.01g) immediately after harvest.
Bulb diameter (mm): Two perpendicular measurements to largest horizontal diameter of the bulb were taken with digital calipers, and the bulb diameter was recorded as an average of the two measurements.
Fleshy leaves count: The count of fully formed fleshy scales in each bulb was recorded by carefully dissecting the bulb.
Dry matter content: For this analysis, 50 g of each bulb (fleshy leaves) were placed in the oven (at 65°C) until a constant weight was achieved (approximately after 72 hours). Dry matter percentage was recorded as: final dry weight / fresh weight × 100.
Bioactive compounds analysis
Fresh onion bulbs were washed up, peeled and cut into little fragments. The samples were then immediately frozen in liquid nitrogen and later lyophilized. The dried samples were milled fine and pulverized with a mechanical mill and stored at -20°C until use.
Total phenolic content (TPC): TPC was determined using the Folin-Ciocalteu method [15]. Sample extraction was performed using Soxhlet apparatus with 80% ethanol at 55°C for 4 hours. The extract was concentrated under reduced pressure using rotary evaporator. Phenolic content was determined by mixing 150 μL extract with 500 μL Folin-Ciocalteu reagent and 1.5 mL 20% sodium carbonate. The samples were incubated in complete darkness for 2 hours at ambient temperature and light absorbance was measured at 765 nm using UV-Vis spectrophotometer. Results were expressed as mg gallic acid equivalent per gram dry weight (mg GAE/g DW).
Total flavonoid content (TFC): TFC was quantified using aluminum chloride colorimetric method with rutin as standard [16]. Previously described methanolic extract (50 μL) was mixed with 1 mL methanol, 4 mL distilled water, 0.3 mL 20% sodium nitrite, and 0.3 mL 20% aluminum chloride. After 10 minutes, 2 mL 1M sodium hydroxide was added, volume adjusted to 10 mL, and light absorbance measured with a UV-VIS spectrophotometer at 510 nm. Results were then expressed as mg rutin equivalent per gram dry weight (mg RE/g DW).
Glycoside content (GC): The overall glycoside concentration was determined based on the Baljet colorimetric assay [17]. The dried sample of an onion (10 g) was extracted with 80% methanol over a 24-hour period at room temperature. The extract was then combined (10 mL of the extract and 5 mL of Baljet reagent, which was prepared as 95 mL of 1% picric acid and 5 mL of 10% NaOH) and stored to react for 1 hour in the dark. Then, 20 mL of distilled water was added and the absorbance was read at 495 nm with a UV-VIS spectrophotometer. The securidaside (isolated cardiac glycoside of Securigera securidaca) was used as a reference to construct standard curve and the measurements were converted to a percentage in dry weight (% DW).
Terpenoid content: The spectrophotometric approach was employed to determine the total terpenoid content [18]. The dried sample (1.5 g) was mixed with 7 mL of methanol-acetonitrile solution (1:1 v/v), swirled and stored in the darkness of 24 hours. The mixture was centrifuged at 6000 rpm for 10 minutes and 5 mL of the supernatant was transferred and reacted with 1.5 mL of chloroform and 0.5 mL of concentrated sulfuric acid. The absorbance at a wave length of 538 nm was determined after color development with linalool serving as a standard. The terpenoids were then estimated as percentage in dry weight (% DW).
Saponin content: The amount of total saponins was measured on the basis of vanillin sulfuric acid colorimetric assay [19] to reduce the interference of solvents. In a nutshell, the dried sample of 0.25 g was extracted using 80% ethanol (20 mL, at 60°C, for 1 hour), filtered, and reduced to a known volume. An aliquot (0.25 mL) was dried, 0.25 mL of 8% vanillin solution and 2.5 mL of 72% H2SO4 were added. The mixture was vortex-mixed, and the sample was then heated at 60°C to eliminate moisture. The absorbance value was taken at 544 nm and the total saponins were estimated as percentage in dry weight compared to an aescin equivalent (% DW).
Statistical analysis
Data were subjected to analysis of variance (ANOVA) using SAS statistical software (Version 9.4). Treatment means were compared using Duncan’s Multiple Range Test (DMRT) at p<0.05 significance level. Interaction effects between iron and potassium treatments were evaluated, and correlation analysis was performed to assess relationships between yield and quality parameters. Standard errors were calculated for all means to assess treatment variability.
Results and Discussion
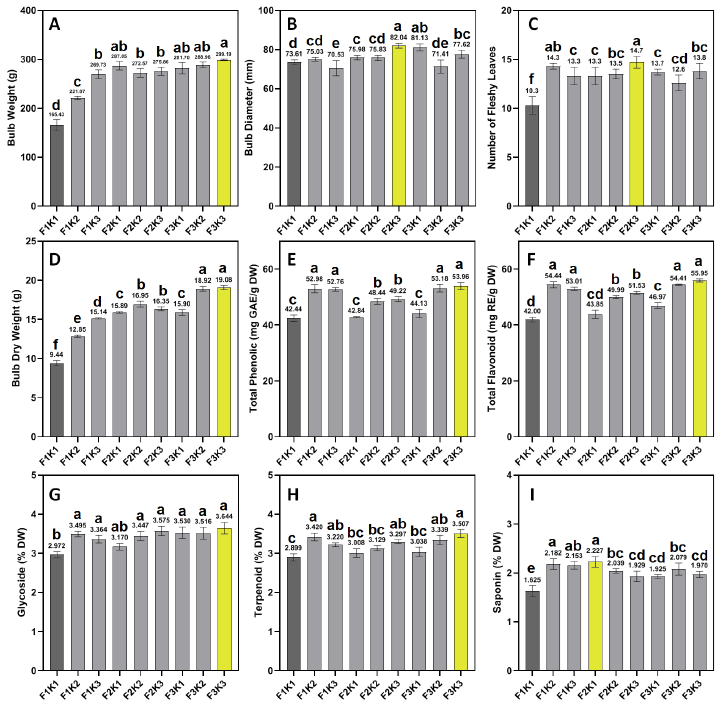
The results show that bulb weight increased progressively with higher iron and potassium inputs. The F3K3 treatment (4.5 g/L Fe-EDTHA + 300 kg/ha K₂SO₄) produced the heaviest bulbs at 299.19 g, representing an 80.9% increase over the control (F1K1: 165.43 g). Even the lowest effective treatment, F1K2 (no iron, 150 kg/ha K), yielded a 33.6% increase (221.07 g). Notably, the combination of F2K3 (2.25 g/L Fe-EDTA + 300 kg/ha K) achieved 288.96 g, which is 74.7% higher than control (nearly matching F3K3) (Fig. 1 A). Therefore, a decent increase can be observed under K fertilization only; however, the combined application of chelated iron and potassium resulted in more pronounced increases in bulb weight.
As for bulb diameter, The F2K3 treatment resulted in the highest values (82.04 mm) significantly surpassing other treatments except for F3K1. On the other hand, the control recorded the smallest bulb diameter value with 73.61 mm (Fig. 1 B). Similarly, a significantly higher number of fleshy leaves was observed in F2K3 (14.7 leaves per bulb) while the lowest number of fleshy leaves was observed in the control with 10.3 leaves per bulb (Fig. 1 C). Dry weight corresponded with fresh weight values, as both F3K3 and F3K2 scored the highest values (19.08 and 18.92 g, respectively). Both treatments surpassed all other treatment combinations. It is worth noting that F3K3 resulted in 102% increase in DW compared to control, while F3K2 [4.5 g/L chelated iron (Fe-EDTHA)+150 kg/ha K₂SO₄] resulted in 25% increase compared to F1K3 (No chelated iron application and 300 kg/ha K₂SO₄) which highlights the synergistic effect of Fe and K at these levels in the build and accumulation of dry matter (Fig. 1 D).
The analysis of bioactive compounds revealed a non-significant synergistic effect between Fe and K applications. Total phenolic content (TPC), Total flavonoid content (TFC), Glycosides content, and Terpenoids content were maximized under F3K3 with 53.96 mg GAE/g DW, 55.95 mg RE/g DW, 3.644% DW, and 3.507% DW, respectively. Notable differences were observed between this treatment and control in terms of the aforementioned compounds, as F1K1 (control) scored significantly lower values (Fig. 1 E-H). However, no significant differences were noted between F3K3 and other application treatments (F1K2, F1K3, and F3K2). This observation indicated that K influence on bulb content of phenols, flavonoids, glycosides, and terpenoids was more pronounced compared to Fe. On the other hand, the highest saponin content was observed in F2K1 (2.25 g/L chelated iron (Fe-EDTA) and 0 kg K/ha), which highlights Fe role in saponin synthesis (Fig. 1 I).
The outstanding positive changes marked in this study in terms of bulb fresh and dry weight could be ascribed to various interlinked physiological processes that are boosted under the synergistic effect of chelated iron and potassium nutrition. Application of chelated iron strongly enhances photosynthetic activity by increasing chlorophyll production and electron transfer activities, where iron is considered an important building block of cytochromes, ferredoxin, and iron-sulfur complexes vital in functioning of photosystems [20][21]. These findings are in accordance with reports showing that increases in bulb weight were directly proportional to the increases in carbon assimilation capacity [22].
The alkaline conditions of the experimental site (pH 7.8-8.2) reflect common problems encountered in onion production in semi-arid regions where the precipitation of iron significantly reduces its availability. Under these conditions, the chelated form of iron is necessary to ensure sufficient nutrition, and the effectiveness of chelated iron used in this study confirms its functionality under field conditions. High soil alkalinity also impacts potassium availability by competitive ion behavior and fixation which can severely reduce availability. Increasing potassium rates in the current study, especially K3 levels (144 kg K₂O/ha), improved bulb fresh and dry weights compared to control and to lower K rates. For instance, F1K3 (No chelated iron application and 300 kg/ha K₂SO₄) resulted in 22% and 63% increases in bulb fresh weight compared to F1K2 (No chelated iron application and 150 kg/ha K₂SO₄) and F1K1 (control), respectively. Similarly, F1K3 resulted in 17.8% and 60.3% increases in bulb dry weight compared to F1K2 and control, respectively. However, foliar application of 4.5 g/L chelated iron (Fe-EDTHA) without any potassium addition (F3K1) resulted in a comparable bulb weight to that of F3K3 (with 300 kg/ha K₂SO₄). Similarly, no significant differences were observed between F3K2 [4.5 g/L chelated iron (Fe-EDTHA)+150 kg/ha K₂SO₄] and F3K3 in terms of bulb dry weight. These observations collectively highlight the importance of synergistic effect of Fe and K for onion production under alkaline soil conditions. Moreover, these results might refer to the role of chelated Fe in enhancing K absorption and utilization in plant under alkaline soil conditions [23].
The substantial increases in phenolic compounds reflect enhanced activity throughout the phenylpropanoid pathway where iron serves as a cofactor for several key enzymes [24], while potassium maintains optimal cellular conditions for enzyme function [8]. Flavonoid synthesis involves multiple iron-dependent enzymes, including flavanone 3-hydroxylase and flavonol synthase [25], with the 33% increase in flavonoid content indicating enhanced activity of these enzymes under chelated iron application, while potassium maintains the cellular pH optimal for flavonoid stability and transport [12]. Therefore, the significant enhancement of bioactive compound accumulation—particularly phenolics and flavonoids—in onion bulbs following chelated iron and potassium application underscores the pivotal role these nutrients play in modulating secondary metabolism. Given that phenolic and flavonoid compounds are well-established mediators of plant stress responses (e.g., oxidative, biotic, and abiotic stress mitigation), their upregulation suggests that Fe and K fertilization may bolster the plant’s physiological resilience. Concurrently, this metabolic shift elevates the nutraceutical and nutritional quality of the bulbs, reinforcing the agronomic and functional benefits of integrated Fe–K nutrient management.
The observed yield increases (80.9%) have provided clear economic backing to the full-nutrition approach, since the increased investment expended can be regained in terms of marketable yield after the season. Furthermore, the elevated levels of bioactive compounds enhance the functional quality of onion bulbs, aligning them with the increasing demands of health-conscious consumer markets, where such value-added attributes increasingly command premium pricing.
Despite the notable importance of the current observations, several limitations should be acknowledged: (1) the study was conducted over a single growing season, limiting assessment of year-to-year variability; (2) only one onion cultivar was evaluated, potentially limiting generalizability across genetic backgrounds; (3) economic analysis was not conducted to assess cost-effectiveness across different market conditions. Consequently, future investigations should address long-term sustainability of the nutrition approach across multiple seasons; optimization for different onion cultivars and genetic backgrounds; integration with organic production systems; post-harvest storage quality and shelf-life implications; and the environmental impact assessment of chelated micronutrient applications.
Conclusions
It can be concluded that higher potassium input (144 kg K₂O/ha) is necessary under alkaline conditions in order to achieve a notable increase in onion bulb weight. However, similar outcomes are achievable with minimum potassium fertilization when applying foliar chelated iron (4.5 g/L Fe-EDTHA). Moreover, both applications showed a notable impact on bulb content of phenols, flavonoids, glycosides, and terpenoids. Consequently, more research should be focused on the extended application of chelated iron under various K inputs in order to fully understand and exploit the potential of the synergistic relationship between these two elements in onion nutrition.
Conflict of interest statement
The authors declared no conflict of interest.
Funding statement
The authors declared that no funding was received in relation to this manuscript.
Data availability statement
The authors declared that the experimental data will be available upon reasonable request from the corresponding author.
References
- FAOSTAT. Crops and Livestock Products. FAO. 2023. Available From: Link
- Vuković S, Moravčević D, Gvozdanović-Varga J, Dojčinović B, Vujošević A, Pećinar I, Kilibarda S, Kostić AŽ. Elemental Profile, General Phytochemical Composition and Bioaccumulation Abilities of Selected Allium Species Biofortified with Selenium under Open Field Conditions. Plants. 2023;12(2):349. DOI
- Singh N, Gusain A, Nigam M, Mishra AP. The pharmacological and therapeutic versatility of Allium species: a comprehensive exploration of bioactive constituents and biological activities. Discov. Appl. Sci. 2025;7(4):349. DOI
- Dobermann A, Bruulsema T, Cakmak I, Gerard B, Majumdar K, McLaughlin M, Reidsma P, Vanlauwe B, Wollenberg L, Zhang F. Responsible plant nutrition: A new paradigm to support food system transformation. Glob. Food Secur. 2022;33:100636. DOI
- Ahmed N, Zhang B, Chachar Z, Li J, Xiao G, Wang Q, Hayat F, Deng L, Narejo M, Bozdar B. Micronutrients and their effects on Horticultural crop quality, productivity and sustainability. Sci. Hortic. 2024;323:112512. DOI
- Manthey JA, Tisserat B, Crowley DE. Root responses of sterile‐grown onion plants to iron deficiency1. J. Plant Nutr. 1996;19(1):145-61. DOI
- López-Rayo S, Nadal P, Lucena JJ. Reactivity and effectiveness of traditional and novel ligands for multi-micronutrient fertilization in a calcareous soil. Front. Plant Sci. 2015;6:752. DOI
- Hasanuzzaman M, Bhuyan M, Nahar K, Hossain M, Mahmud J, Hossen M, Masud A, Moumita, Fujita M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy. 2018;8(3):31. DOI
- Abu El-Azm N, Metwally A, Abd Elhady S. Onion Bulb Yield and Quality as Influenced by Seed or Seedling Planting Methods As well as Potassium Fertilizer Forms, K2SO4 and KCl, and Their Combination. Sci. J. Agric. Sci. 2023;5(1). DOI
- Salama DM, Khater MA, Abd El-Aziz ME. The influence of potassium nanoparticles as a foliar fertilizer on onion growth, production, chemical content, and DNA fingerprint. Heliyon. 2024;10(11):e31635. DOI
- Rietra RPJJ, Heinen M, Dimkpa CO, Bindraban PS. Effects of Nutrient Antagonism and Synergism on Yield and Fertilizer Use Efficiency. Commun. Soil Sci. Plant Anal. 2017;48(16):1895-920. DOI
- Islam MM, Li L, He J, Naznin A, Huda S, Ahemd T, Tissue D, Chen Z. Decoding Plant Metabolomic Response to Potassium and Nutrient Stresses in Controlled Environments. Physiol. Plant. 2025;177(5):e70547. DOI
- Mohammadnia S, Haghighi M, Mozafarian M, Geösel A. Impact of Mycorrhiza Inoculations and Iron Amino Chelate on Growth and Physiological Changes of Cucumber Seedlings Across Different pH Levels. Plants. 2025;14(3):341. DOI
- El-Gioushy SF, Ding Z, Bahloul AME, Gawish MS, Abou El Ghit HM, Abdelaziz AMRA, El-Desouky HS, Sami R, Khojah E, Hashim TA. Foliar Application of Nano, Chelated, and Conventional Iron Forms Enhanced Growth, Nutritional Status, Fruiting Aspects, and Fruit Quality of Washington Navel Orange Trees (Citrus sinensis L. Osbeck). Plants. 2021;10(12):2577. DOI
- Sokolovska V, Jankulovska MS. Folin–Ciocâlteu Method for Determination of Total Polyphenols in Onion. J. Agric. Food Environ. Sci. 2022;76(2):12-9. DOI
- Masood S, Rehman AU, Ihsan MA, Shahzad K, Sabir M, Alam S, Ahmed W, Shah ZH, Alghabari F, Mehmood A. Antioxidant potential and α-glucosidase inhibitory activity of onion (Allium cepa L.) peel and bulb extracts. Brazilian Journal of Biology 2023;83:00264. DOI
- Maheshwaran L, Nadarajah L, Senadeera SPNN, Ranaweera CB, Chandana AK, Pathirana RN. Phytochemical Testing Methodologies and Principles for Preliminary Screening/ Qualitative Testing. Asian Plant Res. J. 2024;12(5):11-38. DOI
- Łukowski A, Jagiełło R, Robakowski P, Adamczyk D, Karolewski P. Adaptation of a simple method to determine the total terpenoid content in needles of coniferous trees. Plant Sci. 2022;314:111090. DOI
- Le AV, Parks SE, Nguyen MH, Roach PD. Improving the Vanillin-Sulphuric Acid Method for Quantifying Total Saponins. Technologies. 2018;6(3):84. DOI
- Okla MK, Saleem MH, Saleh IA, Zomot N, Perveen S, Parveen A, Abasi F, Ali H, Ali B, Alwasel YA. Foliar application of iron-lysine to boost growth attributes, photosynthetic pigments and biochemical defense system in canola (Brassica napus L.) under cadmium stress. BMC Plant Biol. 2023;23(1):648. DOI
- Sheng Y, Cheng H, Wang L, Shen J, Tang M, Liang M, Zhang K, Zhang H, Kong Q, Yu M. Foliar Spraying with Compound Amino Acid‐Iron Fertilizer Increases Leaf Fresh Weight, Photosynthesis, and Fe‐S Cluster Gene Expression in Peach (Prunus persica (L.) Batsch). Biomed Res. Int. 2020;2020(1):2854795. DOI
- Wood JB, Cramer CS, Steiner R, Heerema R, Schutte BJ, Guzman I. Onions Selected for Reduced Symptom Expression of Iris Yellow Spot Have Higher Photosynthetic Rates. HortScience. 2023;58(3):254-8. DOI
- Guo G, Xiao J, Jeong BR. Iron Source and Medium pH Affect Nutrient Uptake and Pigment Content in Petunia hybrida ‘Madness Red’ Cultured In Vitro. Int. J. Mol. Sci. 2022;23(16):8943. DOI
- Connorton JM, Balk J, Rodríguez-Celma J. Iron homeostasis in plants – a brief overview. Metallomics. 2017;9(7):813-23. DOI
- Halbwirth H, Fischer TC, Schlangen K, Rademacher W, Schleifer K, Forkmann G, Stich K. Screening for inhibitors of 2-oxoglutarate-dependent dioxygenases: Flavanone 3β-hydroxylase and flavonol synthase. Plant Sci. 2006;171(2):194-205. DOI
Cite this article:
Abdullah M.A., Allawi A.H., Ahmed T.S.. Chelated iron and potassium sulfate synergistically improve bulb yield and levels of bioactive phytochemicals in onion grown on alkaline soils. DYSONA-Applied Science. 2026;7(1):134-141. doi: 10.30493/DAS.2025.012411

