Bassam M. Ewhayid 1; Majid A. Ibrahim 2*; Eman M. Abdulzahra 1
1, Department of Biology, University of Basrah, Iraq
2, Department of Horticulture and Landscape, College of Agriculture, University of Basrah, Iraq
E-mail:
majid.abdulhameedl@uobasrah.edu.iq
Received: 30/11/2022
Acceptance: 21/02/2023
Available Online: 22/02/2023
Published: 01/07/2023

Manuscript link
http://dx.doi.org/10.30493/DAS.2023.374878
Abstract
This study was conducted with the aim of moringa tree propagation in the presence of chitosan and some growth regulators using tissue culture technique. The stem nodule explants of moringa tree were cultured on Murashige and Skoog medium supplemented with different combinations of benzyl adenine (0.5, 1, and 1.5 mgL-1) and chitosan (0, 5, and 10 mgL-1) in addition to naphthalene acetic acid at a constant concentration of 0.2 mgL-1 for shoot proliferation. Rooting formation on the formed shoots was investigated using different concentrations of Naphthalene acetic acid (0.5, 1, and 1.5 mgL-1). The results showed that the medium supplemented with 5 mgL-1 chitosan had a significant increase in the response of the stem nodule to the shoot proliferation (78.22%) and shoot numbers (5.12). On the other hand, supplementing the medium with 1 mgL-1 benzyl adenine led to a shoot proliferation response of 68.33% and 4.91 shoots per explant. The combination of 5 mgL-1 chitosan and 1.5 mgL-1 benzyl adenine resulted in the highest response to the proliferation of shoots (91.33%). While the combination of 5 mgL-1 chitosan and 1 mgL-1 benzyl adenine resulted in an increased shoot production with 6.73 shoots per explant. The rooting medium supplemented with 1.5 mgL-1 naphthalene acetic acid was significantly superior in terms of shoots response to root formation (71.70%), number of roots per explant (6.67), and root length (3.50 cm). The current results highlight the advantages of including chitosan in the proliferation and rooting medium of moringa.
Keywords: Chitosan, Moringa, Shoot proliferation, Benzyl adenine, Naphthalene acetic acid
Introduction
Moringa (Moringa oleifera L.) tree is one of the plants that humans have been interested in since ancient times. Belonging to the family Moringaceae, this plant is cultivated as ornamental trees or windbreaks [1]. Additionally, various parts of this plant are nutritionally and medicinally important. Its leaves and seeds have been used as an integrated food for generations in many regions in Asia and Africa since these plant parts are proven to contain large amounts of antioxidants, vitamins, amino acids, carbohydrates, and important elements such as iron, potassium, phosphorous, calcium, zinc, and selenium [2][3]. Furthermore, moringa leaves are rich in many bioactive compounds that are of medical importance, such as flavonoids, including quercetin, myricetin, and kaempferol, which reduce the risk of cancer and some heart and circulatory diseases [4][5]. It also contains antioxidants and vitality against bacteria, fungi, and viruses, as it has been proven that it can have an anti-COVID-19 effect [6]. All these uses resulted in moringa becoming an economically important plant, with increased concentration on its cultivation and multiplication methods.
Chitosan is a crude carbohydrate polymer extracted from the acetyl group of chitin by deacetylation, which includes N-acetyl-D-glucosamine residues and D-glucosamine that are linked together by a ß-1,4-glycoside linkage [7]. When applied to a culture medium, chitosan can operate as a bio stimulator, stimulating plant tissue growth and serving as an antioxidant and antimicrobial substance. Therefore, positive results were obtained when utilizing chitosan under in vitro conditions with the aim of shoot proliferation [8-10]. An experiment of adding different concentrations of chitosan to tiger orchid (Grammatophullum speciosum) culture medium showed that a concentration of 5-15 mgL-1 led to the highest average number of shoots [11]. Additionally, a combination of 120 mgL-1 chitosan and 0.5 mgL-1 benzyl adenine (BA) resulted in the highest mean of M26 apple rootstock proliferation after 12 weeks of culturing.
Several studies have been conducted on the micropropagation of moringa with the aim of shoot proliferation. These researches showed that the addition of benzyl adenine to the nutrient media on which the explants were grown in vitro, led to an increased shoot formation [13-16]. Considering the promising results of utilizing chitosan in culture media and the economic importance of moringa plant, this study was conducted to evaluate the effectiveness of using chitosan as a growth stimulator beside other growth regulators in moringa shoot proliferation and rooting.
Materials and Methods
Plant material preparation
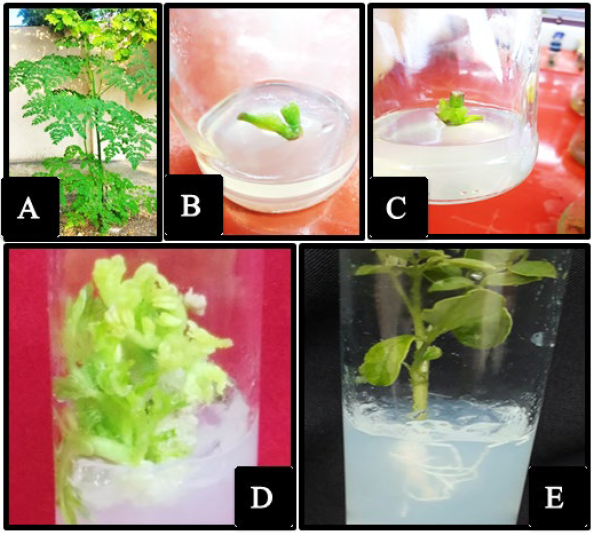
The study was carried out in the plant tissue culture laboratory of Fadak Company, Basrah governorate, Abi Al-Khasib district. The explants containing stem nodules were taken from trees aged 1-2 years (Fig.1 A). Then they were placed in an antioxidant solution consisting of 100 mgL-1 ascorbic acid, and 150 mgL-1 citric acid for 24 hours. Explants were sterilized using Clorox solution at 6% NaOCl and Tween 20 drops for 20 minutes, and then washed with sterile distilled water 4 times.
Culture media
Shoot proliferation medium was prepared using Murashige and Skoog (MS) medium [17] with 4.43 g MS salts (HIMEDIA, India) per liter. Plant growth regulators were added with different combinations of cytokinin (benzyl adenine) at 0.5, 1, and 1.5 mgL-1 in addition to naphthalene acetic acid at a constant concentration of 0.2 mgL-1. Chitosan (Sigma-Aldrich Company, USA) was dissolved by 3-4 drops of diluted acetic acid (1 N) at rates of 0, 5, and 10 mgL-1.
The regenerated shoots (10-15 cm) rooting characteristics were tested by culturing on MS medium supplemented with 0.5, 1, and 1.5 mgL-1 naphthalene acetic acid and a constant concentration of chitosan (5 mgL-1).
The pH in both media of was adjusted to 5.8 using 0.1 N of NaOH or 0.1 N of HCl. Then 8 gL-1 of agar was added to the MS medium. Then the MS medium was poured into culture jars. Then the culture jars were sterilized with an autoclave at a temperature of 121°C and a pressure of 1.04 kg cm-2 for 20 minutes.
Measurements
The data relating to the studied traits in both experiments were recorded after four weeks of cultivation:
- Response of explant to shoot proliferation (% in each replicate)
- Shoot numbers per explant
- Response of shoot for root formation (% in each replicate)
- Root numbers per shoot
- Root length (cm)
Experimental design and statistical analysis
A factorial experimental design was used in the shoot proliferation experiment while the simple experiment of rooting was carried out in a completely randomized design. Each treatment in both experiments was repeated ten times with ten cultures in each replicate. The data were analyzed statistically using analysis of variance. Statistical analysis was carried out using the statistical program Genestat version 14. The comparison between the means of the factorial treatments was done using the least significant difference test at 1% probability level [18].
Results
Shoot proliferation
Chitosan had a significant positive effect on the response of the stem nodules to shoot proliferation and shoot numbers of moringa after four weeks of culturing (Tables 1 and 2). The MS medium supplemented with 5 mgL-1 chitosan had a significant increase in the response of the stem nodule to the shoot proliferation and shoot numbers scoring 78.22% and 5.12 shoots per explant, respectively. On the other hand, the chitosan-free medium (chitosan control) recorded the lowest shoot proliferation response (48.44%), while MS medium supplied with chitosan at 10 mgL-1 recorded the lowest value in shoot numbers (3.12 shoots per explant).
As for benzyl adenine (BA) content, it was evident that 1 mgL-1 treatment resulted in a significantly higher shoot proliferation response rate in cultures (68.33%) and a higher number of shoots (4.91 shoots per explant). On the other hand, the lowest response of the stem nodule to shoot proliferation (48.44%) and the number of shoots (3.60 shoots per explant) were observed in media containing 0.5 mgL-1 BA.
The combination effect of chitosan and benzyl adenine significantly affected the response of the stem nodules to the proliferation of shoots. The combination of 5 mgL-1 chitosan and 1.5 mgL-1 benzyl adenine was superior in terms of shoot proliferation response (91.33%). While chitosan-free MS medium supplemented with 0.5 mg L-1 BA recorded the lowest response to the proliferation of shoots at 31.67% (Table 1). On the other hand, the combination of 5 mgL-1 chitosan and 1 mgL-1 BA resulted in the formation of the highest number of shoots (6.73 shoots per explant), while the lowest number (2.20 shoots per explant)was observed in the cultures of 10 mgL-1 chitosan and 1 mgL-1 BA medium.
Rooting shoots
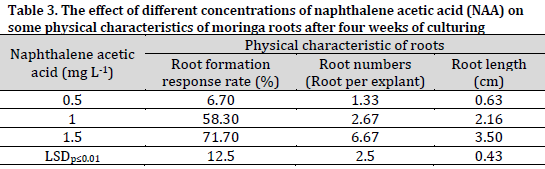
The results showed that naphthalene acetic acid content in culture medium had a significant effect on the rooting of moringa shoots after four weeks of culturing (Fig. 1 E) (Table 3). The treatment of 1.5 mgL-1 naphthalene acetic acid was significantly superior in terms of shoot response to root formation (71.70%), number of roots (6.67 roots per shoot), and root length (3.50 cm). On the other hand, the lowest root initiation rate (6.7%), number of roots (1.33 roots per shoot), and root length (0.63 cm) were recorded in the rooting medium containing 0.5 mgL-1 of naphthalene acetic acid.
Discussion
The current results showed that chitosan might have a positive influence on shoot proliferation response rate and shoot numbers of moringa stem nodules cultures. The cause of the shoot number increase can be attributed to chitosan’s capability of activating and promoting the meristematic tissues toward cell division and growth of lateral buds in the cultured explant, resulting in an overall growth improvement and enhancing vegetative traits, including the shoot numbers [19]. Furthermore, chitosan might contribute to the biosynthesis pathway of endogenous auxin, since it promotes the formation of tryptophan, the precursor product of auxin [9]. However, shoot formation response differs based on chitosan concentrations in the culture medium depending on the nature of plant species and explant source [10].
The cause for the lower rate of response to the shoot formation with increased concentrations of benzyl adenine might be attributed to the disturbance in the balance between exogenous and endogenous hormones in these tissues under in vitro culture, which led to a reduced shoot formation response [20][21]. On the other hand, BA concentration at 1 mgL-1 was optimal for BA function in reducing apical dominance while inducing cell division and differentiation in axillary buds [22-24]. Therefore, the balance between the hormonal role of BA and the bio-stimulating influence of chitosan resulted in an enhanced shoot formation response rate. On the other hand, the increase in rooting response rate in the medium with high NAA content is attributed to the key role played by auxins in cell division, growth, and development [25-27]. Including chitosan as a bio-stimulant in rooting media might have enhanced the overall NAA effect over rooting response rates in addition to root length and number, since chitosan is known to assist the increase of biomass and root length in multiple plant species. However, since only one chitosan concentration was used in the current study (5 mg.L-1), more experiments are needed to confirm this positive effect and optimize chitosan content in moringa rooting media.
Conclusion
The current study shows the advantages of including chitosan in the stem nodules propagation medium of Moringa oleifera. An optimal concentration of chitosan (5 mgL-1) and benzyl adenine (1 mgL-1). Were found to induce the highest proliferation response rate and highest number of shoots after four weeks of culturing. Additionally, MS medium supplied with 1.5 mgL-1 of naphthalene acetic acid and 5 mgL-1 of chitosan led to the highest rooting response.
References
| 1 | Poteet MD, Number U. Biodiesel crop implementation in Hawaii. Honolulu: Prepared by the Hawaii Agriculture Research Center. Aiea, HI for State of Hawaii Department of Agriculture. 2006:89. |
| 2 | Fozia F, Meenu R, Avinash T, Abdul AK, Shaila F. Medicinal properties of Moringa oleifera: An overview of promising healer. J. Med. Plant Res. 2012;6(27):4368-74. DOI |
| 3 | Yadav R, Khare RK, Singhal A. Qualitative phytochemical screening of some selected medicinal plants of shivpuri district (mp). Int. j. life-sci. sci. res. 2017;3(1):844-7. DOI |
| 4 | Abdulkarim SM, Long K, Lai OM, Muhammad SK, Ghazali HM. Some physico-chemical properties of Moringa oleifera seed oil extracted using solvent and aqueous enzymatic methods. Food Chem. 2005;93(2):253-63. DOI |
| 5 | Ibrahim M. Role of Endogenous and Exogenous Hormones in Bioactive Compounds Production in Medicinal Plants Via In Vitro Culture Technique. In Plant Hormones: Recent Advances, New Perspectives and Applications. Intechopen. 2022:131. DOI |
| 6 | Colunga Biancatelli RM, Berrill M, Catravas JD, Marik PE. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front. Immunol. 2020:1451. DOI |
| 7 | Chibu H, Shibayama H. Effects of chitosan applications on the growth of several crops. In Chitin and Chitosan in Life Science. Kodansha Scientific LTD. 2001;892:305-16. |
| 8 | Charoenwattana PI, Petprapai UM. Effects of chitosan and Lotus extracts as growth promoter in Dendrobium orchid. Int. J. Environ. Rural Dev. 2013;4(2):133-7. DOI |
| 9 | Youssef SM. Chitosan and thidiazuron improve regeneration efficiency of strawberry (Fragaria x ananassa Duch.) cv. Festival from different explant types. Middle East J. 2016;5(4):856-67. |
| 10 | Safana HS, Ibrahim MA, Abd AM. Impact of chitosan and benzyl adenine on shoot multiplication of kumquat plant (Citrus japonica Thumb.) in vitro. Int. J. Agricult. Stat. Sci. Vol. 2022;18(1):359-65. |
| 11 | Sopalun K, Thammasiri K, Ishikawa K. Effects of chitosan as the growth stimulator for Grammatophyllum speciosum in vitro culture. Int. J. Biotechnol. Bioeng. 2010;4(11):828-30. DOI |
| 12 | Dastjerd ZH, Jabbarzadeh Z, Marandi RJ. Interaction effects of chitosan, benzyladenine, and gibberellic acid on in vitro proliferation of M26 apple rootstock. Hortic. Environ. Biotechnol. 2013;54(6):538-47. DOI |
| 13 | Fatima H, Perveen A, Qaiser M. Micropropagation to rescue endangered plant Moringa concanensis Nimmo (Moringaceae). Pak. J. Bot. 2016;48(1):291-4. |
| 14 | Alshwerf AO, Amin LS, Ibrahim F, Youssef J. Protective Effect of Moringa oleifera Extract on Experimentally LPS-induced periodontitis. Int. J. Adv. Res. 2017;5:1734-40. DOI |
| 15 | Drisya Ravi RS, Siril EA, Nair BR. The effect of silver nitrate on micropropagation of Moringa oleifera Lam. an important vegetable crop of tropics with substantial nutritional value. Physiol. Mol. Biol. Plants. 2019;25(5):1311-22. DOI |
| 16 | Khierallah HS, Al-Obaidy OM. Effect of explant type and some plant growth regulators on culture initiation of stevia plants in vitro. Iraqi J. Agric. Sci. 2017;48(5):1206-14. DOI |
| 17 | Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15(3):473-97. DOI |
| 18 | Al-Rawi KM, Khalaf Allah AM. Design and analysis of agricultural experiments. El Mousel Univ., Iraq. 2000;19:487. |
| 19 | Palpandi C, Shanmugam V, Shanmugam A. Extraction of chitin and chitosan from shell and operculum of mangrove gastropod Nerita (Dostia) crepidularia Lamarck. Int. J. Med. Med. Sci. 2009;1(5):198-205. |
| 20 | Khalifa NS. Using tissue culture technology in palm propagation. King Abdulaziz Library for Technical Sciences, National Center for Agricultural Technology, King Abdulaziz City for Technical Sciences, Saudi Arabia. 2011:56. |
| 21 | Ibrahim MA, Al-Taha HA, Saaid ZA. Propagation of strawberry via in vitro adventitious shoot formation technique. Iraqi J. Agric. Sci. 2013;44(1),69-80. |
| 22 | Taiz L, Zeiger E, Møller IM, Murphy A. Plant physiology and development. Sinauer Associates Incorporated; 2015. |
| 23 | Ioio RD, Linhares FS, Scacchi E, Casamitjana-Martinez E, Heidstra R, Costantino P, Sabatini S. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol.. 2007;17(8):678-82. DOI |
| 24 | Hamad MS, Al-Jubouri MT. Effect of BAA and NAA on the micropropagation of citrus rootstocks in vitro. Euphrates J. Agric. Sci. 2014;6,48-56. |
| 25 | Ibrahim MA, Sabty MZ, Mussa SH. In vitro culture of big-sage (Lantana camara L.) plant. Acta Sci. Pol. Hortorum Cultus. 2020;19(2),67-73. DOI |
| 26 | Ibrahim MA, Yassin MM. Direct shoot regeneration by in vitro culture of the gerbera (Gerbera jamesonii Bolus) capitulum explants. Plant Cell Biotechnol. Mol. Biol. 2020;(49&50)31:1-9. |
| 27 | Ibrahim MA, Jasim AM, Abbas MF. Somatic embryogenesis and plantlet regeneration in Indian jujube (Ziziphus mauritiana Lamk.) cv. Zaytoni. Genet. Plant Physiol. 2011;1(3/4):150-4. |
| 28 | Luan LQ, Ha VT, Nagasawa N, Kume T, Yoshii F, Nakanishi TM. Biological effect of irradiated chitosan on plants in vitro. Biotechnol. Appl. Biochem. 2005;41(1):49-57. DOI |
Cite this article:
Ewhayid, B., Ibrahim, M., Abdulzahra, E. Chitosan as a growth stimulator of moringa (Moringa oleifera L.) under in vitro conditions. DYSONA – Applied Science, 2023; 4(2): 28-34. doi: 10.30493/das.2023.374878
