Amani Fitori 1*; Belgasim M. Abdulnabi 1; Ramadan A. S. Ali 1; Sayed Mohamed Ali 1
1, Department of Marine Sciences, Omar Al-Mukhtar University, Albaida, Libya
E-mail:
amanifitori1@gmail.com
Received: 20/04/2020
Acceptance: 10/06/2020
Available Online: 10/06/2020
Published: 01/07/2020

Manuscript link
http://dx.doi.org/10.30493/dls.2020.224289
Abstract
Effects of heavy metals [copper (Cu), zinc (Zn), chromium (Cr), nickel (Ni), manganese (Mn), iron (Fe), Aluminum (Al), cadmium (Cd), mercury (Hg), stannum (Sn), and lead (Pb)] present in Tubruk harbor (polluted site) comparing to Umm Hufayan lagoon water (control) on liver and kidney performance of Mugil cephalus captured from both sites were investigated. For this purpose, surface water samples of both chosen sites were compared in terms of heavy metals concentrations, pH, and dissolved oxygen. Mullet fish were captured from both sites and blood samples were taken from the fish and sent to the lab for liver and kidney function tests. The results showed that heavy metals levels were significantly higher in Tubruk than those in Umm Hufayan lagoon. Additionally, significantly higher total protein and albumin levels were found in the blood serum of M. cephalus of Umm Hufayan, compared to Tubruk harbor fish samples; however, higher levels of bilirubin (BR), alanine transaminase (ALT), aspartate transaminase (AST), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), acid phosphatase (ACP), urea, uric acid, creatinine, and glucose were observed in the blood serum of Tubruk fish. Positive correlation was noticed between LDH and Cd levels (r: 0.576, p: 0.02, LDH = 0.000Cd + 0.36, R2 : 0.509) in Tubruk fish, and between AST and Hg of Umm Hufayan fish (r: 0,488, p: 0.055, AST = 0.005Hg +0.497, R2: 0.078).
Keywords: Mugil cephalus, Liver functions, Kidney functions, Heavy metals, Libya, Mediterranean Sea
Abbreviations: Tubruk harbor (TH), Umm Hufayan lagoon (UHL), Alanine aminotransferase (ALT), aspartate aminotransferase (AST), Lactate dehydrogenase (LDH), Alkaline phosphatase (ALP), Acid phosphatase (ACP).
Introduction
Heavy metals are elements with atomic weights ranging between 63.546 and 200.590 and a specific gravity greater than 4.0 [1]. They are classified as essential and non-essential elements [2]. Essential heavy metals are those required in minute quantities by living organisms, e.g. cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), vanadium (V), strontium (Sr), and zinc (Zn); however, excessive levels of these elements are poisonous. Non-essential heavy metals, on the other hand, are toxic even in ultra-trace concentrations, e.g. mercury (Hg), lead (Pb), cadmium (Cd), and arsenic (As). Heavy metals and xenobiotics are regarded as the serious pollutants of the aquatic environment because of their persistence and tendency to bioaccumulation in the environment aquatic organisms, particularly fishes, due to the advanced level they occupy in the aquatic food chain [3].
Heavy metals enter the aquatic ecosystem through atmospheric deposition, geological erosion [4], and human activities such as industrial effluxes, sewage, marine transportation, agriculture, and mining wastes [5][6][7]. The contamination chain in the aquatic environment always starts with industry and other anthropological activities then transferred into the aquatic ecosystem through the atmosphere or soil interactions with water surfaces, these contaminants enter the biomass of small water organisms (phytoplankton, zooplankton) and end in fish which is consumed by animals and humans [8]. Additionally, heavy metals enter the fish body through gills and skin. Furthermore, Heavy metals cannot be detoxicated by metabolic processes and therefore, marine organisms bio-accumulate these elements in their bodies to reach concentrations of 100.000 times the surrounding water such as Hg and Cd levels in oysters and mussels [9]. Heavy metals toxicity can be categorized in three levels as they block the essential functional groups of proteins and enzymes; displace metal ions from biomolecules, or modify the space structure of biomolecules [10] which illustrates the threat of high heavy metals concentration in the marine environment [11][12][13].
Fish resistance to toxicity depends on its physiological state and stress reactions involve various physiological changes including alterations in blood composition and histopathology of various organs particularly haemopoietic and immune organs [14]. Additionally, stressors can also induce diseases outbreaks and cause low productivity in aquaculture [15] and these environmental changes affect fish populations drastically [16]. Complete blood count (CBC) in addition to some liver functions (i.e. Total protein, Albumin, Bilirubin, AST, ALT, LDH, ALP, ACP in the blood) and some kidney functions (i.e. Urea, Uric acid, Creatinine levels) are among the highly influenced pollution bio-indicators [17].
Fish are undoubtedly key members of many natural food webs and considered important sources of animal proteins in the human diet (16-23%) [18]. Studies on fishes have assumed greater significance due to the increasing emphasis on pisciculture and the growing community awareness of the ecological impact of natural fresh and marine water resources [19]. Additionally, monitoring environmental, physiological, and pathological changes can provide basic information regarding the status of open-water fish communities within their environment [20]. Therefore, this research aimed to:
Evaluate the concentrations of heavy metals (Cd, Cr, Ni, Pb, Hg, Cu, Fe, Mn, and Zn) in Tubruk harbor water (polluted site) and Umm Hufayan lagoon (control).
Investigate the effects of these pollutants on liver and kidney functions of mullet fish (Mugil Cephalus) present in both sites.
Materials and Methods
The study sites
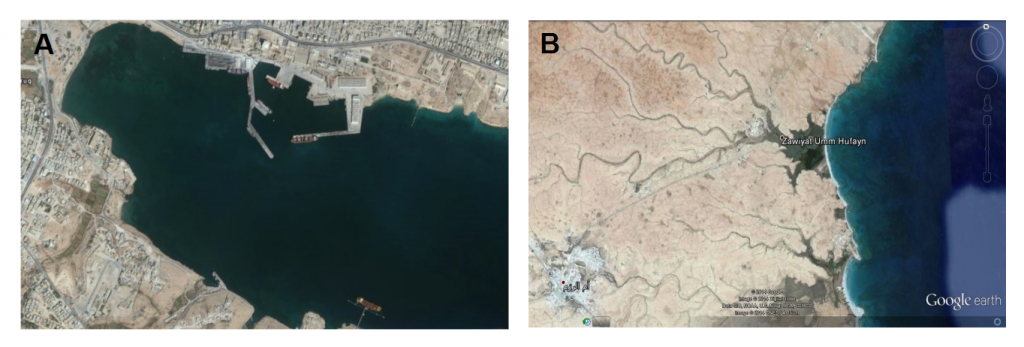
Tubruk harbor (TH) (Fig. 1 A) is an important natural commercial and fishing harbor with a terminal for oil shipping. Additionally, Tubruk is a populous industrial city and the municipal untreated sewage water of the city is discharged directly into the sea. Furthermore, precipitation on the many garbage dumping cites within the city eventually infiltrates into the sea, leading to aquatic pollution on the coast of this harbor.
Umm Hufayan lagoon (UHL) (Fig. 1 B) is a brackish lagoon that covers a surface area of about 2 Km2 with depth ranges between 0.5 and 3m. Underground springs at the southern side of the Lagoon discharge fresh water into it. Therefore, the aquatic environment of the lagoon is healthy and the anthropogenic use is limited to a small artisanal fishery [21].
Sampling
Water samples
Sixteen water samples (2-3 liters each) were collected from each of TH and UHL at about 20 cm below the sea surface between September 2017 and April 2018 at a rate of two samples per month. The samples were first filtered in the field to remove particulates, stored in clean polyethylene bottles, and kept in a refrigerator until analysis.
Fish samples
A hundred fresh M. cephalus were collected concomitantly with water sampling from fishers of TH and UHL (50 fish per site). Blood samples were rapidly drawn from the fish caudal vessel or hearts using sterile syringes fitted with 21-gauge needles [22] and were then transferred into conventional blood venojects.
Experimental procedures
Water properties
Water pH and dissolved oxygen were measured directly and simultaneously with water sampling in both sites. pH was defined by pH meter while dissolved oxygen was measured using an oxygen meter.
Determination of heavy metals in the water samples
The digestion of the water samples was carried out according to [23]. 1000 ml of water sample of each location was evaporated until the volume became 100 ml. 15ml HCL (37%) was added and heated on hot plate for 10 min. After cooling, 15ml of HNO3 was added and the digest was again heated until the volume became 50ml. Then, the digested solution was transferred to a 100 ml volumetric flask and brought to a volume of 100 ml with deionized water. Heavy metals in the digested samples were then measured using inductively coupled plasma atomic emission spectroscopy (ICP-OES).
Liver and kidney performance
liver function is commonly estimated through measuring total protein, albumin, bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), and acid phosphatase (ACP) in blood while kidney functions through Urea, Uric acid, Creatinine levels [17].
To obtain serum, blood samples were allowed to clot on ice for 1 h. Serum was then separated from by centrifugation at 14000 g for 5 min [22]. The venojects were then taken to the medical laboratory in the city of Al-Baydafor the determination of the aforementioned indices.
Statistical Analysis
The statistical analysis was performed using SPSS. The two fish groups were compared using Student’s t-test. A difference was considered significant at p<0.05. Additionally, Pearson’s correlation test was performed to study the relation between the concentration of heavy metals and liver enzymes.
Results
Physicochemical characteristics of study sites
The pH differences between the two sites were minimal; however, dissolved oxygen levels were lower in TH than in UHL in the study duration (Table 1).
Heavy metals concentrations
Water samples analysis in both sites illustrated that heavy metals presented in the following order in TH Fe (24.7ppm) > Pb (3.3ppm) > Zn (3.1ppm) > Cu (1.06ppm) > Ni (0.87ppm) > Cr (0.37ppm) > Cd (0.32ppm) > Hg (0.60ppm) >Mn (0.14ppm), while in UHL, heavy metals presented in the following order Fe (16.3ppm) > Zn (2.30ppm) > Cu (0.25ppm) > Cr (0.027ppm) > Ni (0.08ppm) >Mn (0.03ppm) > Cd (0.001ppm) >Pb (0.001ppm) > Hg (0.001ppm). Concentrations of all metals were significantly higher in TH than in UHL except for Mn, Zn, and Fe (Table 2).
Biomarkers of fish samples
Mean total length (T.L) and total weight (T.W) of Mugil cephalus were 20.04 cm and 98.73 g respectively in TH and 20.52 cm and 86.32.21 g in UHL with no significant differences observed between the two locations (Table3).
Liver and kidney performance indicators
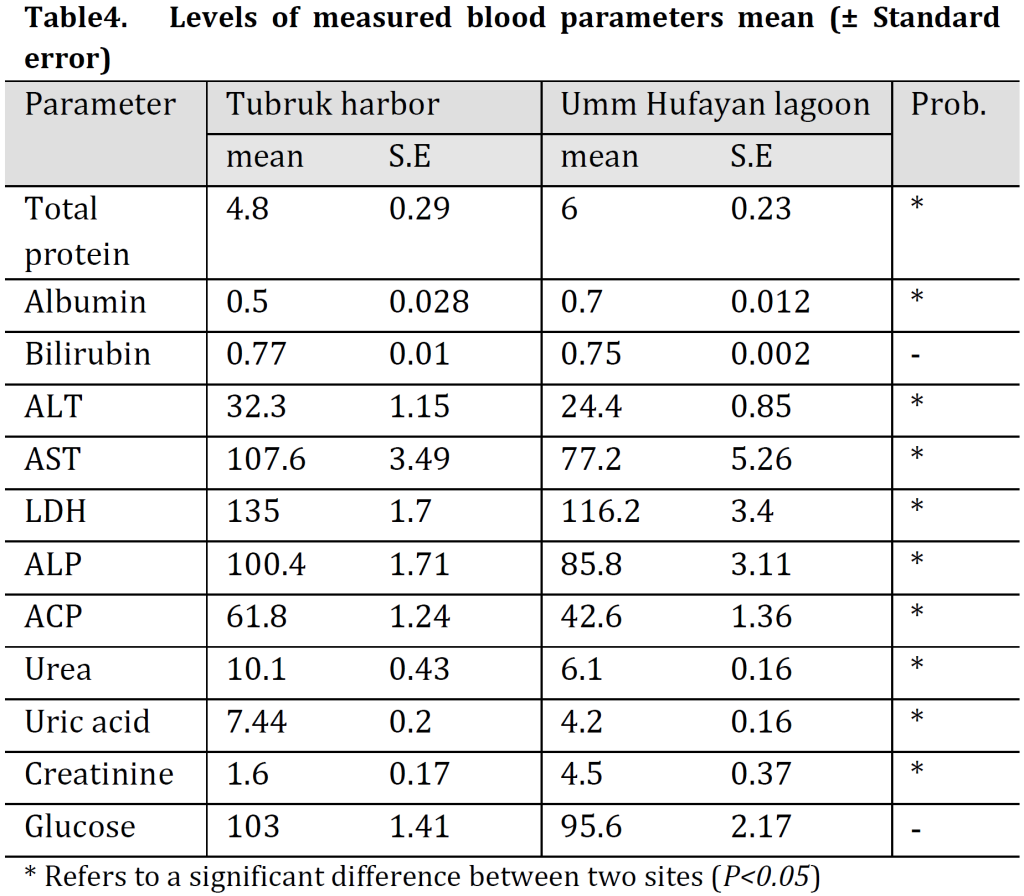
Significantly higher total protein, albumin, and creatinine levels were found in blood serum of Mugil cephalus from UHL comparing to TH samples; however, higher ALT, AST, LDH, ALP, ACP, urea, and uric acid levels were observed in blood serum of TH’s samples. No significant differences in bilirubin or glucose levels were observed between the two locations (Table 4).
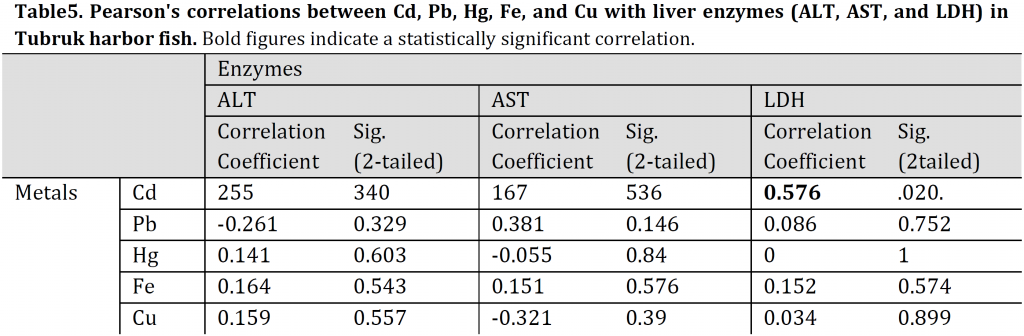
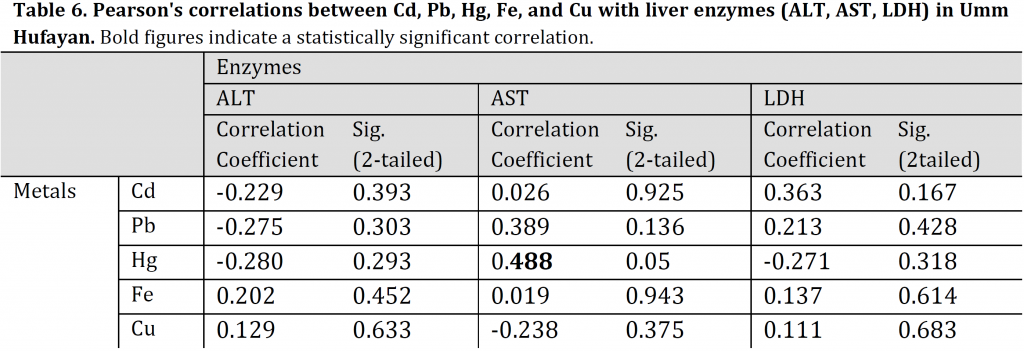
Correlations between heavy metals concentrations and levels of the liver enzymes
Pearson’s correlations of Cd, Pb, Hg, Fe, and Cu with liver enzymes (ALT, AST, LDH) of TH fish indicated a significantly positive correlation between LDH and Cd (r: 0.576, p: 0.02). The linear regression was: LDH = 0.000Cd + 0.36, R2: 0.509. Additionally, a moderate significantly negative correlation was also observed between AST and Hg for UHL fish (r: 0,488, p: 0.055). The linear regression was: AST = 0.005Hg +0.497, R2: 0.078) (Tables 5, 6). All the other TH and UHL correlations were not significant.
Discussion
The results of total protein and albumin in serum showed a significant drop in fish of TH (polluted site) comparing to UHL (control). This drop was accompanied by a significant increase in water’s Pb, Zn, Cu, Ni, Hg, and Cd in TH suggesting that the elevated levels of these heavy metals might have had an inhibition effect on the protein production process [24]. Hypoproteinemia was previously observed by [25] in hybrid striped bass after chronic exposure to stress. Additionally, [26] reported that hypoproteinemia and hyperproteinemia may be observed in fish as a stress response. [27] observed a decrease in total protein content in blood serum in addition to a decrease in body weight of Clarias gariepinus fish supplemented with a diet containing 15 ppm mercuric oxide for 4 weeks.
The present study showed a slight but non-significant increase in bilirubin levels in fish of TH compared to UHL fish. This increase may be due to liver malfunction, as [28] previously reported a marked increase in bilirubin in the wild Don salmon exposed to paper mill and field drain discharges. Additionally, hyperbilirubinemia jaundiced condition was reported to be the final stage of pollutant intoxication [29]. Furthermore, hepatotoxic agents were reported to induce bilirubinaemia in wild Don salmon and captive North Esk salmon [28].
In the present study, a significant increase in ALT, AST, ALP, and LDH was documented in TH fish samples compared to those of UHL. This is in agreement with [30] who observed a significant increase in ALT and AST in Clarias gariepinus fish serum when mercuric oxide was included in the dietary program. The elevated levels of urea and liver enzymes in attribution to the high levels of heavy metals were consistent with the results of [31] who reported liver and kidney injury of Tilapia zilli after exposure to cadmium chloride at 0.25 ppm for 21 days. Furthermore, [27] found an increase in urea levels in Clarias lazera fish after as a result of vanadium toxicity. Urea was also significantly increased in Channa punctatus fish exposed to nonlethal concentrations of As2O3 for 14 days [32]. However, the current study contradicts [27][31][32] reports of creatinine increase with heavy metals exposure as the serum of UHL fish contained significantly higher levels of creatinine when compared to TH (polluted site).
An increase in glucose level was observed in the serum of TH fish compared to UHL; however, this increase was not significant. In general, blood glucose level is highly influenced by carbohydrate metabolism rate under stress and hypoxia conditions as [33] previously reported that hyperglycemia occurs when a stress stimuli is followed by a rapid glucocorticoids secretion from the adrenal tissue. The effects of sub-lethal concentration of nickel on the freshwater fish Oreochromis niloticus were studied by [34] and it was reported that serum ALT and AST levels in addition to serum glucose were significantly increased under this treatment, while the total protein and albumin levels were decreased.
In the present study, cases of no correlation, negative correlation, and positive correlation were observed between heavy metal levels in water and liver enzyme efficiency in Mugil cephalus. Such varied responses may be due to the properties of the metal, concentration, toxicity, absorption, metabolism, and detoxification methods in addition to fish species, age, size, and food habits [35][36]. The observed lack of correlation between some liver enzymes and some heavy metals may be due to a low concentration of these metals below the level at which the enzymes respond [37].
The statistically significant correlation observed between Cd concentration and LDH activity was in correspondence with [38] results who reported that Cd increased concentration was the cause of elevated LDH in Cyprinus carpio.
References
| 1 | Connell DW, Miller GJ. Chemistry and ecotoxicology of pollution. John Wiley & Sons; 1984. |
| 2 | Storelli MM, Storelli A, D’addabbo R, Marano C, Bruno R, Marcotrigiano GO. Trace elements in loggerhead turtles (Caretta caretta) from the eastern Mediterranean Sea: overview and evaluation. Environ. Pollut. 2005;135(1):163-70. DOI |
| 3 | Has-Schön E, Bogut I, Strelec I. Heavy metal profile in five fish species included in human diet, domiciled in the end flow of River Neretva (Croatia). Arch. Environ. Contam. Toxicol. 2006;50(4):545-51. DOI |
| 4 | Gümgüm B, Tez Z, Gülsün Z. Heavy metal pollution in water, sediment and fish from the Tigris River in Turkey. Chemosphere. 1994;29(1):111-6. DOI |
| 5 | Tarvainen T, Lahermo P, Mannio J. Sources of trace metals in streams and headwater lakes in Finland. Water, Air, Soil Pollut. 1997;94(1-2):1-32. DOI |
| 6 | Haynes D, Johnson JE. Organochlorine, heavy metal and polyaromatic hydrocarbon pollutant concentrations in the Great Barrier Reef (Australia) environment: a review. Mar. Pollut. Bull. 2000;41(7-12):267-78. DOI |
| 7 | Islam MS, Tanaka M. Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar. Pollut. Bull. 2004 Apr 1;48(7-8):624-49. DOI |
| 8 | Kádár I, Koncz J, Fekete S. Experimental study of Cd, Hg, Mo, Pb and Se movement in the soil-plant-animal system. Krmiva: Časopis o hranidbi životinja, proizvodnji i tehnologiji krme. 2001;43(4):185-90. |
| 9 | Klake RK, Nartey VK, Doamekpor LK, Edor KA. Correlation between heavy metals in fish and sediment in Sakumo and Kpeshie Lagoons, Ghana. J. Environ. Prot.. 2012;3(09):1070. DOI |
| 10 | Langston WJ. Toxic effects of metals and the incidence of metal pollution in marine ecosystems. Heavy metals in the marine environment. 1990:101-22. |
| 11 | Uluturhan E, Kucuksezgin F. Heavy metal contaminants in Red Pandora (Pagellus erythrinus) tissues from the eastern Aegean Sea, Turkey. Water Res. 2007 ;41(6):1185-92. DOI |
| 12 | Naji A, Ismail A, Ismail AR. Chemical speciation and contamination assessment of Zn and Cd by sequential extraction in surface sediment of Klang River, Malaysia. Microchem. J. 2010;95(2):285-92. DOI |
| 13 | Bashir FH, Othman MS, Mazlan AG, Rahim SM, Simon KD. Heavy metal concentration in fishes from the coastal waters of Kapar and Mersing, Malaysia. Turkish J. Fish. Aquat. Sci. 2013;13(2):375-82. DOI |
| 14 | Ololade IA, Oginni O. Toxic stress and hematological effects of nickel on African catfish, Clarias gariepinus, fingerlings. J. Environ. Chem. Ecotoxicol. 2010 Mar 31;2(2):014-9. |
| 15 | Allen P. Changes in the haematological profile of the cichlid Oreochromis aureus (Steindachner) during acute inorganic mercury intoxication. Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology. 1994;108(1):117-21. DOI |
| 16 | Rose KA, Cowan Jr JH, Houde ED, Coutant CC. Individual-based modeling of environmental quality effects on early life stages of fish: A case study using striped bass. Oak Ridge National Lab., TN (United States); 1992. |
| 17 | Kayode SJ, Shamusideen SA. Haematological studies of Oreochromis niloticus exposed to diesel and drilling fluid in Lagos, Nigeria. Int J Biodiv Conserv. 2010;2(5):130-3. |
| 18 | Oshode OA, Bakare AA, Adeogun AO, Efuntoye MO, Sowunmi AA. Ecotoxicological assessment using Clarias gariepinus and microbial characterization of leachate from municipal solid waste landfill. Int. J. Environ. Res., 2(4): 391-400. |
| 19 | Kulkarni R, Bedjargi P. Serum Biochemical Parameters of Four Fresh Water Indian Carps from a Local Aquatic Body. Int. j. innov. stud. aquat. biol. fish. 2016;2(3):15-20. DOI |
| 20 | Elnabris KJ, Muzyed SK, El-Ashgar NM. Heavy metal concentrations in some commercially important fishes and their contribution to heavy metals exposure in Palestinian people of Gaza Strip (Palestine). J. Assoc. Arab Univ. Basic Appl. Sci. 2013;13(1):44-51. DOI |
| 21 | Abdalhamid AH, Ramadan AA, Mohamed E, Sayed MA, Elawad AN. Study of some ecological and biological parameters on European eel Anguilla anguilla in Umm Hufayan brackish lagoon, Eastern Libya Mediterranean Sea. Bulletin de l’Institut Scientifique, Rabat. 2018(40):23-30. |
| 22 | Hrubec TC, Robertson JL, Smith SA. Effects of temperature on hematologic and serum biochemical profiles of hybrid striped bass (Morone chrysops x Morone saxatilis). Am. J. Vet. Res. 1997;58(2):126-30. |
| 23 | Salim R. Effect of storage on the distribution of trace elements (lead,’cadmium, copper, zinc and mercury) in natural water. J. Environ. Sci. Health A. 1987;22(1):59-69. DOI |
| 24 | Abdulnabi BM. Xenobiotics and the susceptibility of Oreochromis niloticus (Lineaus, 1757) from Lake Maryut to environmental interactions and hepatic biotransformations. (M.Sc Thesis, Alexandria University). 2002. |
| 25 | McDonald DG, Reader JP, Dalziel TR. The combined effects of pH and trace metals on fish ionoregulation. In Acid toxicity and aquatic animals. Cambridge University Press Cambridge. 1989:221-42 |
| 26 | Adham KG, Hamed SS, Ibrahim HM, Saleh RA. Impaired functions in Nile Tilapia, Oreochromis niloticus (Linnaeus, 1757), from polluted waters. Acta Hydrochim. Hydrobiol. 2001;29(5):278-88. DOI |
| 27 | Zaki MS, Sharaf NE, Osfor MH. Effect of vanadium toxicity in Clarias lazera. J Am Sci. 2010;6(12):291-6. |
| 28 | Groman DB, Miller K. Haemolytic anaemia of wild Atlantic salmon: haematology and chemical pathology. Aquaculture. 1987;67(1/2):210-2. DOI |
| 29 | Everall NC, Mitchell CG, Groman DB, Johnston JA. Tracing of haematotoxic agents in water with the aid of captive fish: a study with captive adult Atlantic salmon Salmo salar in the river Don Aberdeenshire, Scotland. Dis. Aquat. Org. 1991;10(2):75-85. |
| 30 | Zaki MS, Nabila E, Fawzi OM, Awad I, Nagwa SA. Effect of mercuric oxide toxicity on some biochemical parameters on African cat fish Clarias gariepinus present in the River Nile. Life Sci J. 2011;8(1):363-8. 8: 363-368. |
| 31 | Zaki MS, Fawzi OM, Moustafa S, Seamm S, Awad I, El-Belbasi HI. Biochemical and immunological studies in Tilapia zilli exposed to lead pollution and climate change. Nat Sci. 2009;7:90-3. |
| 32 | Roy S, Bhattacharya S. Arsenic-induced histopathology and synthesis of stress proteins in liver and kidney of Channa punctatus. Ecotoxicol. Environ. Saf. 2006;65(2):218-29. DOI |
| 33 | Abbas WT, AboHegab EA, Bashter AE, Tawfik MA. Fish as an indicator for pollutants in aquatic environment (Doctoral dissertation, Ph.D. Thesis, Zoology Department, Faculty of Science, Cairo University, Egypt). 2006. |
| 34 | Al-Attar AM. The influences of nickel exposure on selected physiological parameters and gill structure in the teleost fish, Oreochromis niloticus. J. Biol. Sci.. 2007;7(1):77-85. |
| 35 | Abedi Z, Hasantabar F, Khalesi MK, Babaei S. Enzymatic activities in common carp; Cyprinus carpio influenced by sublethal concentrations of cadmium, lead, chromium. World Journal of Fish and Marine Sciences. 2013;5(2):144-51. |
| 36 | Ibrahim NA, El-Naggar GO. Assessment of heavy metals levels in water, sediment and fish at cage fish culture at Damietta Branch of the river Nile. J. Egypt. Acad. Environ. Develop. 2006;7(1):93-1114. |
| 37 | Nemcsok J, Benedeczky I, Boross L, Asztalos B, Orban L. Subcellular localization of transaminase enzymes in fishes and their significance in the detection of water pollution. Acta Biol. Szeged. 1981;27(9):15. |
| 38 | Baghshani H, Shahsavani D. Effects of lead acetate exposure on metabolic enzyme activities in selected tissues of common carp (Cyprinus carpio). Comp Clin Path. 2013;22(5):903-7. DOI |
Cite this article:
Fitori, A., Abdulnabi, B., Ali, R., Ali, S. Effects of some heavy metal pollutants on liver and kidney performance of mullet captured from Tubruk harbor comparing to Umm Hufayan lagoon. DYSONA – Life Science, 2020;1(2): 83-90. doi: 10.30493/dls.2020.224289
