Hasan H. Mahdi 1; Saadi M. Al-Ghrairi 1*; Rasha A. Musa 1
1, Soil and Water Resources Center, Agricultural Research Directorate, Ministry of Science and Technology, Baghdad, Iraq
E-mail:
sadialgreary@yahoo.com
Received: 02/09/2023
Acceptance: 21/11/2023
Available Online: 22/11/2023
Published: 01/01/2024

Manuscript link
http://dx.doi.org/10.30493/DAS.2023.414578
Abstract
Plants cultivated in calcareous soil exhibit phosphorus deficiency, resulting in diminished crop productivity and reduced yield quality. In order to enhance phosphorus availability in soil, the introduction of organic acids represents a viable strategy. Consequently, a controlled incubation experiment was carried out to investigate the impact of organic acid supplementation on phosphorus availability in calcareous soils. The experiment involved the addition of three organic acids (citric acid, ascorbic acid, and tartaric acid) at varying amounts (15, 25, and 35 mmol/kg soil) to a calcareous soil supplemented with phosphate fertilizer (diammonium phosphate) at a constant level of 50 kg P/ha. The soil samples were subjected to incubation at a temperature range of 17-20°C at field capacity moisture content for varying durations of 15, 30, 45, and 60 days. The measurements of accessible phosphorus, total phosphorus, and soil pH were conducted following each incubation period. The findings of the study indicated a significant rise in the concentration of available phosphorus and a corresponding decrease in total phosphorus and pH levels throughout the experiment when organic acids were introduced. Among the organic acids tested, citric acid exhibited the most favorable outcomes, particularly at a concentration of 35 mmol/kg soil resulting in the highest phosphorus availability. The administration of organic acids may have contributed to an increased buildup of phosphorus in its organic form. Hence, the utilization of organic acid has the potential to augment the accessibility of phosphorus in calcareous soils and alter the balance between mineralization and immobilization, favoring the enrichment of the organic fraction of phosphorus rather than the relatively more stable mineral calcium phosphates. This has the potential to improve the long-term availability of phosphorus in calcareous soils.
Keywords: Organic acids, Available phosphorus, Calcareous soils, Citric acid, Phosphate
Introduction
Phosphorus is a vital essential nutrient for the growth and development of plants. The concentration of phosphorus in soil is influenced by various factors, including the quality and quantity of certain components such as clay minerals, oxides, organic matter, and calcium carbonate [1]. The availability of phosphorus (P) is adversely impacted by alkaline pH levels exceeding 7.5. At such elevated pH values, phosphate ions exhibit a tendency to rapidly react with calcium (Ca) and magnesium (Mg), resulting in the formation of less soluble compounds. On the other hand, under conditions of low pH, phosphate ions undergo reactions with aluminum (Al) and iron (Fe) to yield compounds that are comparatively less soluble [2]. The accessibility of phosphorus in soil to plants is contingent upon the replenishing of labile P in the soil from other P fractions [3]. However, maintaining a pH range of 6 to 7 in the soil is essential for ensuring optimal availability of phosphorus.
The introduction of phosphate fertilizer is expected to undergo a short-term conversion to sodium hydroxide-P (NaOH-P), followed by a longer-term conversion to calcium phosphate (Ca-P) and citrate bicarbonate dithionite-P (CBD-P) [4]. Calcareous soil is characterized by the substantial presence of free excess lime, specifically calcium or magnesium carbonate. Lime exhibits solubility in soil with a pH ranging from neutral to acidic, while its solubility is limited in alkaline soil. Thus, lime functions as a reservoir for the precipitation of calcium phosphate on the soil surface in alkaline soils aggravating the issue of phosphorus availability [5]. Phosphorus stabilization in calcareous soil leads to restricted phosphorus availability, which subsequently causes phosphorus deficiency and an overall decrease in yield quality and quantity.
Soil is known to contain a variety of organic acids, such as tartaric, fumaric, malic, citric, and ferulic acids [6]. These organic acids primarily originate from plants, microorganisms, and organic waste in the soil [7]. The presence of these acids in the soil has significant implications for the chemical transformations of phosphorus, including the dissolution of phosphate compounds and the mineralization of organic phosphorus [8]. Organic acids are naturally synthesized by plant roots and are commonly found in soil environments [9]. For instance, soil solution surrounding the roots of chickpea and lupine plants exhibited concentrations of organic acids at 84 mmol and 58 mmol, respectively [10]. The release of organic acids and anions from plant roots can enhance the solubility of certain stable soil phosphorus pools [11]. This process is of great importance in terms of soil phosphorus availability and the potential loss of phosphorus from soil to water [12].
The low phosphorus availability is a significant challenge in agricultural production systems located in arid and semi-arid regions. Therefore, the objective of the present study is to evaluate the potential impact of incorporating three different organic acids (citric acid, ascorbic acid, and tartaric acid) at varying concentrations (15, 25, and 35 mmol/kg) on the levels of available and total phosphorus in surface calcareous soil samples obtained from the experimental station of AL-Latifia, located in Baghdad, Iraq.
Materials and Methods
Soil samples
A soil incubation experiment was done at the Soil and Water Resources Center, Agricultural Research Directorate, Ministry of Science and Technology in Iraq. Soil samples from the surface layer (0-30 cm) were obtained from the experimental station located in AL-Latifia, Baghdad, Iraq. The soil samples were subjected to air-drying and subsequent sieving with a 2 mm mesh size. Subsequently, the samples were thoroughly mixed and 400 grams of soil were placed into plastic pots with a volumetric capacity of 500 cm3.
Organic acids addition
Three different organic acids (citric acid, ascorbic acid, and tartaric acid) were introduced into the pots at varying amounts of 0 (control), 15, 25, and 35 mmol/kg. Additionally, diammonium phosphate was applied to all pots as a phosphate fertilizer at a rate of 50 kg P h-1 (1 g/kg soil). The experiment was conducted in a randomized complete block design (RCBD) with three replications of each treatment. The soil samples were subjected to incubation at a temperature range of 17-20°C for durations of 15, 30, 45, and 60 days. The moisture content of the pots was kept at field capacity throughout the incubation period.
Chemical analysis
The Olsen technique was utilized to measure the concentration of available phosphorus [13]. The total phosphorus content was determined using the 60% perchloric acid digestion method [14]. The original soil sample underwent chemical and physical analysis [14] as can be seen in Table 1. Field capacity was calculated using a 1/3-bar pressure plate extractor. The concentration of ammonium and nitrate (NH4+NO3) was determined utilizing a 2M KCl solution by the utilization of a Kjeldahl digestor (Vapodest 45s). The quantity of potassium was determined utilizing an ammonium acetate solution (NH4CH3CO2) through the technique of atomic absorption. The concentrations of the elements copper (Cu), manganese (Mn), zinc (Zn), and iron (Fe) were analyzed using a diethylenetriaminepentaacetic acid (DTPA) solution and the atomic absorption spectrometer (Analytik Jena novAA 400P). The gravimetric approach was employed to determine the percentage of calcium carbonate (CaCO3%) through the measurement of carbon dioxide loss. The electrical conductivity (EC) and pH values were determined using a 1:2.5 solution and specialized EC and pH meters. The determination of organic carbon was conducted using the original method of Walkley and Black [15]. The classification of soil texture was conducted using the hydrometer method [16].
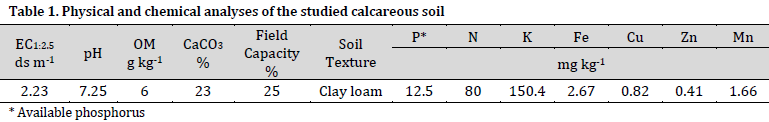
Statistical analysis
The experimental results were analyzed using SPSS software. Confidence interval (CI 95%) test was used to compare means.
Results and Discussion
Impact of organic acids on available phosphorus
The experimental findings indicated that the application of citric acid at concentrations of 25 and 35 mmol/kg soil led to a notable enhancement in the availability of phosphorus during the course of the study, in comparison to the control group. At the 15-day mark of the experiment, it was observed that neither of the administrations of ascorbic acid led to a statistically significant enhancement in phosphorus availability. Nonetheless, the administration of ascorbic acid at concentrations of 25 and 35 mmol/kg soil resulted in a considerable enhancement of accessible phosphorus levels following 45 and 60 days of treatment. In a similar manner, it was observed that all three doses of tartaric acid led to elevated amounts of accessible phosphorus following a 45-day incubation period (Fig. 1 A). The application of citric acid at concentrations of 25 and 35 mmol/kg soil demonstrated higher levels of phosphorus availability compared to control (average of 201.25 mg/kg P) and other treatments, resulting in available phosphorus concentrations of 260.45 and 289.33 mg/kg P, respectively.
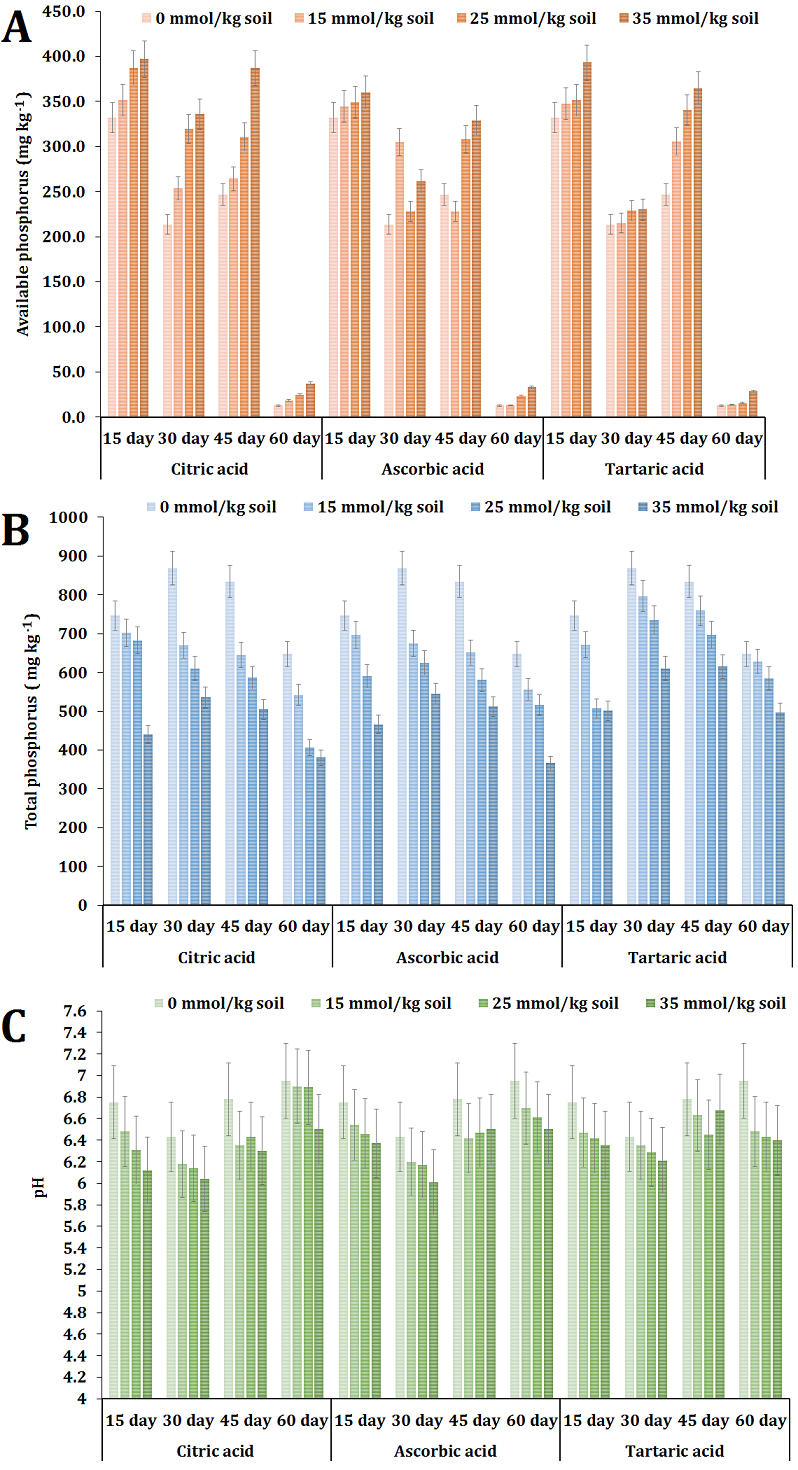
Consistent trends were noted across all samples in relation to the presence of accessible phosphorus throughout the duration of the experiment, irrespective of the kind and concentration of acid used. A significant drop in the concentration of accessible phosphorus was seen following a 30-day incubation period, followed by a subsequent increase after 45 days. Ultimately, it was observed that all samples exhibited a significant reduction in the concentration of accessible phosphorus over a 60-day incubation period. The decline in available phosphorus may be ascribed to the ongoing leaching process facilitated by irrigation, which involved the addition of water to reach field capacity. Nevertheless, the levels of accessible phosphorus were consistently higher in all treatments including organic acids compared to the control group at corresponding stages. Hence, the presence of organic acids was found to have a favorable impact on the release of phosphorus from its reservoirs during the course of the experiment.
The enhanced availability of phosphorus observed in the present study can be due to the multifunctional (carboxylic) groups present in citric acid. Citric acid contains three carboxylic groups, each of which plays a distinct role in its interactions with mineral elements [17]. It has been previously documented that the efficiency of phosphorus release in calcareous soils is higher with citric acid application in comparison to oxalic and malic acids [18].
Impact of organic acids on total phosphorus
The findings indicated that the control treatment had the highest total phosphorus content. In contrast, it was observed that all samples subjected to organic acids treatment had a decreased total phosphorus level. It is worth noting that the organic acids at a concentration of 35 mmol/kg resulted in the lowest overall phosphorus content. Despite remaining relatively stable throughout the duration of the experiment, the levels of total P exhibited a significant decline in the final observation, which occurred after a 60-day incubation period. This drop was observed irrespective of the treatment applied or its concentration (Fig. 1 B).
Elucidating these data may present a complex task, with the primary rationale for this phenomenon being the alteration of the mineralization-immobilization balance towards immobilization due to the application of organic acid treatments. Mineralization is a biologically mediated process whereby the conversion of organic phosphorus in soil into inorganic phosphorus occurs, facilitated by soil microorganisms. In contrast, immobilization refers to the process by which inorganic phosphorus forms undergo conversion into organic forms, thereafter being assimilated into the living cells of soil microorganisms. The existing method employed for measuring total phosphorus may have been insufficient in capturing organic phosphorus due to the exclusive use of perchloric acid in the measurement process. Consequently, the incorporation of nitric acid (HNO3) in conjunction with perchloric acid was deemed necessary for the purpose of decomposing organic substances.
Several conditions can contribute to immobilization, such as the presence of low pH values [19]. Hence, it is imperative to develop an improved understanding of pH levels within the existing experimental parameters.
Impact of organic acids on soil pH
While the observed variations in pH levels did not exhibit statistical significance in comparison to the control group, the administration of citric, ascorbic, and tartaric acids resulted in the acidification of the soil. The initial pH of the soil in the study was recorded as 7.2 (Table 1). The introduction of organic acids resulted in a reduction in soil pH, which was contingent upon the specific kind and concentration of the acids (Fig. 3 C). The treatment of citric acid, ascorbic acid, and tartaric acid at 35 mmol/kg resulted in an average reduction (throughout the experiment) in pH levels to 6.24, 6.35, and 6.41, respectively. The impact of organic acids was seen to reach its peak over a period of 30 days following application. Hence, it is evident that the utilization of organic acids, particularly citric and ascorbic acid, effectively regulated pH levels to ensure phosphorus availability within the optimal range of pH 6-7. These data provide additional support for the greater availability of phosphorus when organic acids are applied. On the other hand acid application may have attributed to an enhanced accumulation of phosphorus in its organic form [19]. Therefore, the application of organic acids can enhance the availability of phosphorus in calcareous soils and potentially shift the equilibrium between mineralization and immobilization towards increasing the organic fraction of phosphorus, as opposed to the more stable mineral calcium phosphates. This could potentially enhance the long-term availability of phosphorus in calcareous soils.
References
| 1 | McDowell R, Sharpley A, Brookes P, Poulton P. Relationship between soil test phosphorus and phosphorus release to solution. Soil Sci. 2001;166(2):137-49. DOI |
| 2 | Jensen T. Soil pH and the Availability of Plant Nutrients. IPNI Plant Nutrition TODAY. 2010. Available from: Link |
| 3 | Zhang TQ, MacKenzie AF, Liang BC, Drury CF. Soil test phosphorus and phosphorus fractions with long‐term phosphorus addition and depletion. Soil Sci. Soc. Am. J. 2004;68(2):519-28. DOI |
| 4 | Mahdi HH, GÖKMEN F, Uygur V. fertilizer-induced geochemical fractions of phosphorus in fruit orchards. Carpathian J. Earth Environ. Sci. 2023;18(1):139-47. DOI |
| 5 | Hopkins B, Ellsworth J. Phosphorus availability with alkaline/calcareous soil. In Western nutrient management conference. Idaho Falls, ID: University of Idaho. 2005;6(3-4):83-93. |
| 6 | Krzyszowska AJ, Vance GF, Blaylock MJ, David MB. Ion‐chromatographic analysis of low molecular weight organic acids in spodosol forest floor solutions. Soil Science Society of America Journal. 1996;60(5):1565-71. DOI |
| 7 | Stevenson FJ. Humus chemistry: genesis, composition, reactions. John Wiley & Sons. 1994. |
| 8 | Hocking PJ. Organic acids exuded from roots in phosphorus uptake and aluminum tolerance of plants in acid soils. Adv. Agron. 2001:63-97. DOI |
| 9 | Strobel BW. Influence of vegetation on low-molecular-weight carboxylic acids in soil solution—a review. Geoderma. 2001;99(3-4):169-98. DOI |
| 10 | Veneklaas EJ, Stevens J, Cawthray GR, Turner S, Grigg AM, Lambers H. Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil. 2003;248:187-97. DOI |
| 11 | Wu L, Kobayashi Y, Wasaki J, Koyama H. Organic acid excretion from roots: a plant mechanism for enhancing phosphorus acquisition, enhancing aluminum tolerance, and recruiting beneficial rhizobacteria. J. Soil Sci. Plant Nutr. 2018;64(6):697-704. DOI |
| 12 | Gang XU, Hongbo S, Rongfu X, Nie Y, Pei Y, Sun Z, Blackwell MS. The role of root-released organic acids and anions in phosphorus transformations in a sandy loam soil from Yantai, China. Afr. J. Microbiol. Res. 2012;6(3):674-9. |
| 13 | Olsen SR. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Department of Agriculture. 1954. |
| 14 | Page AL, Miller RH, Keeney DR. Methods of soil analysis, part 2. Chemical and microbiological properties. 1982;2:643-98. |
| 15 | Walkley A, Black IA. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37(1):29-38. |
| 16 | Gee GW, Bauder JW. Particle‐size analysis. Methods of soil analysis: Part 1 Physical and mineralogical methods. 1986;5:383-411. |
| 17 | Guppy CN, Menzies NW, Moody PW, Blamey FP. Competitive sorption reactions between phosphorus and organic matter in soil: a review. Soil Research. 2005;43(2):189-202. DOI |
| 18 | Jalali M, Jalali M. Effect of low-molecular-weight organic acids on the release of phosphorus from amended calcareous soils: experimental and modeling. J. Soil Sci. Plant Nutr. 2022;22(4):4179-93. DOI |
| 19 | Arenberg MR, Arai Y. Uncertainties in soil physicochemical factors controlling phosphorus mineralization and immobilization processes. Adv. Agron. 2019;154:153-200. DOI |
Cite this article:
Mahdi, H., Al-Ghrairi, S., Musa, R. Enhancing phosphorus availability in calcareous soil through the incorporation of organic acids. DYSONA – Applied Science, 2024;5(1): 7-12. doi: 10.30493/das.2023.414578
