Mesbahuddin Ahadi 1,2; Rongfang Guo 1*
1, College of Horticulture, Fujian Agriculture and Forestry University, Fuzhou 350002, China
2, Horticulture Department. Faculty of Agriculture, Badakhshan University, Fayzabad, Afghanistan
E-mail:
guorofa@163.com
Received: 21/06/2025
Acceptance: 24/07/2025
Available Online: 26/07/2025
Published: 01/10/2026

Manuscript link
http://dx.doi.org/10.30493/DAS.2025.530918
Abstract
This study investigated the efficiency of anther culture and callus induction in three cauliflower genotypes (Graffiti, Sicilian, and Cheddar) using three basal media (MS, NLN, and B5) supplemented with varying concentrations of plant growth regulators. The highest callus induction rate (26.98%) was observed in the Graffiti genotype, followed by Cheddar (11.58%) and Sicilian (9.60%). Morphological analysis revealed that Graffiti produced friable, embryogenic calli, while Sicilian and Cheddar formed compact structures, suggesting divergent regeneration potential. Among the media compositions, MS medium supplemented with 0.3 mg/L 2,4-D and 2.0 mg/L BAP (MS2) showed superior performance. Additionally, callus proliferation was most effective with 1 mg/L BAP and 3 mg/L 2,4-D (88.60%). These findings highlight the genotype-specific and media-dependent responses in cauliflower anther culture, providing valuable insights for the development of double haploids. Therefore, the optimized protocol can accelerate breeding programs, particularly for stress-responsive genotypes like Graffiti.
Keywords: Anther culture, Cauliflower, Callus induction, Genotype
Introduction
Cauliflower (Brassica oleracea var. botrytis) is an economically significant vegetable crop worldwide, known for its nutritional benefits and tolerance to many climates [1][2]. Cauliflower breeding initiatives, as a crucial element of sustainable agriculture, seek to enhance production, stress resilience, and uniformity. Conventional breeding methods are impeded by the crop’s biannual lifetime and significant heterozygosity, requiring advanced biotechnological strategies to expedite genetic enhancement [3-5].
Anther culture facilitates the development of homozygous doubled haploids (DH) within a single generation, circumventing the 5–7 years necessary for successive self-crossing [6-8]. This approach is commonly employed in Brassica species; however, its efficacy in cauliflower is constrained by genotype-dependent responses and inadequate culture conditions [9-11]. Callus induction rates in B. oleracea generally vary between 5% and 40%, with considerable differences among cultivars [12-14]. Most current techniques are tailored for model genotypes, resulting in insufficient exploration of commercially significant cultivars such as Graffiti and Cheddar [15-17].
The efficacy of anther culture is contingent upon multiple factors. Among these factors, the developmental stage of microspores (from late uninucleate to early binucleate), the composition of basal media, and the balance of growth regulators, such as 2,4-dichlorophenoxyacetic acid (2,4-D) (auxin) and 6-benzylaminopurine (BAP) (cytokinin), are critical determinants [18][19]. Murashige and Skoog (MS) medium is often favored for Brassica anther culture because of its balanced nutritional composition [20][21], while Nitsch and Nitsch (NLN) and B5 media have demonstrated potential in some genotypes [22]. On the other hand, the ideal concentrations of 2,4-D and BAP differ significantly among cultivars, requiring genotype-specific optimization [23-25].
Based on the previous debates, this study aimed to assess the effectiveness of anther culture in three cauliflower genotypes (Graffiti, Sicilian, Cheddar) utilizing MS, NLN, and B5 media augmented with different combinations of 2,4-D and BAP. The development of successful genotype-media model procedures for anther culture can be integrated with practical breeding workflows and DH production in cauliflower.
Material and Methods
Plant materials
Three cauliflower (Brassica oleracea var. botrytis) genotypes, Graffiti, Sicilian, and Cheddar, were selected for the study. Healthy plants were grown under controlled greenhouse conditions at 25±1°C and a 16/8 h (light/dark) photoperiod. Unopened flower buds (3.0–4.0 mm length) were collected from 10 plants per genotype when microspores reached the late uninucleate to early binucleate stage, confirmed by acetocarmine staining.
Anther sterilization and isolation
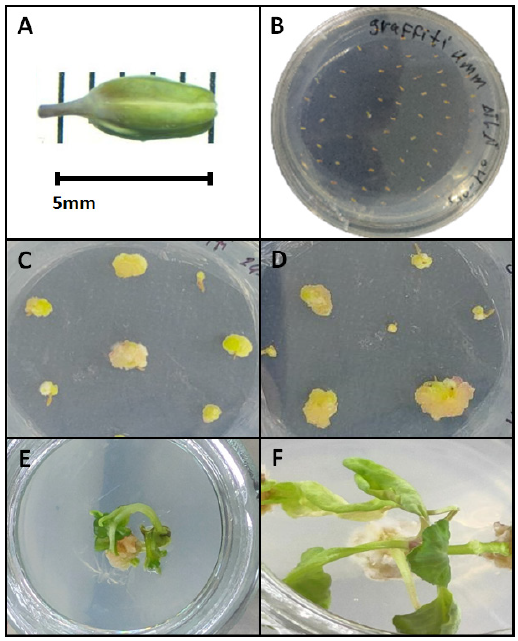
For anther sterilization and isolation, flower buds were surface-sterilized with 70% ethanol for 30 seconds, followed by treatment with 20% commercial bleach (5% sodium hypochlorite) for 10 minutes, and then thoroughly rinsed three times with sterile distilled water to ensure complete removal of disinfectants. Subsequently, anthers were aseptically excised under a stereomicroscope (Fig. 1 A) and carefully transferred into sterile 90 mm Petri dishes containing the prepared culture media (Fig. 1 B).
Culture media and growth regulators
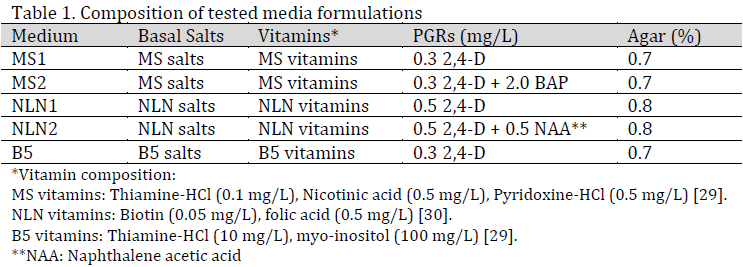
Three basal media were evaluated: Murashige and Skoog (MS) [26], Nitsch and Nitsch (NLN) medium [27], and Gamborg’s B5 medium [28]. Five formulations were tested (Table 1), each containing 3% sucrose (w/v), and solidified with agar (0.7% for MS and B5; and 0.8% for NLN) with pH adjusted to 5.8.
Anther culture and callus induction assessment
Approximately 30–50 anthers per genotype-media combination were cultured in 90-mm Petri dishes (In triplicates), each containing 25 mL of medium (Fig. 1 B). Cultures were maintained in complete darkness at 25±1°C for 4 weeks to induce callus formation (Fig. 1 C and D). At the end of the 4-weeks induction period, the callus induction rate was calculated using the formula:
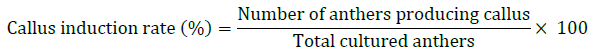
Callus morphology was assessed under a stereomicroscope (Leica MZ6, 40× magnification) and classified into three distinct categories: friable (loose, easily dispersible), compact (dense, nodular), and embryogenic (smooth, globular structures).
Callus proliferation assessment
To determine optimal proliferation conditions, calli from Graffiti genotype were sub-cultured onto MS medium supplemented with four different BAP concentrations (1, 1.5, 2, and 2.5 mg/L), each combined with a constant 3 mg/L 2,4-D. The experiment employed three biological replicates per treatment, with each replicate consisting of 10 calli cultured under controlled conditions (24±1°C, 16/8 h photoperiod). Sub-culturing was performed every 14 days to maintain optimal growth conditions throughout the 4-week evaluation period (Fig. 1 E and F). Then, final proliferation efficiency was quantified at the end of the evaluation period by calculating the proliferation rate:

Moreover, the fresh weight gain of proliferated callus at the end of evaluation period was measured using an analytical balance.
Statistical analysis
For statistical analysis of the experimental data, one-way analysis of variance (ANOVA) was performed using Minitab software followed by post-hoc Fisher’s least significant difference (LSD) test at a significance level of p<0.05 to determine significant differences between treatment means.
Results
Morphological observations
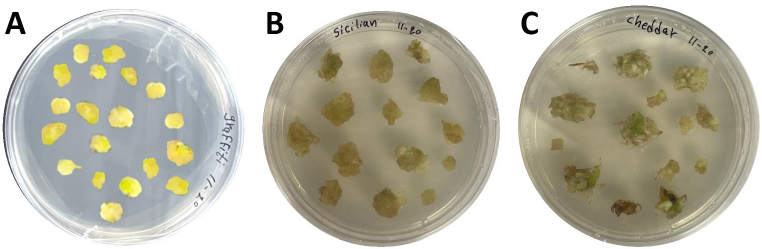
Callus morphology exhibited distinct genotype-specific patterns, with Graffiti producing friable, yellowish embryogenic callus (Fig. 2 A). In contrast, Sicilian and Cheddar formed compact, nodular structures (Fig. 2 B and C).
Media performance in callus induction
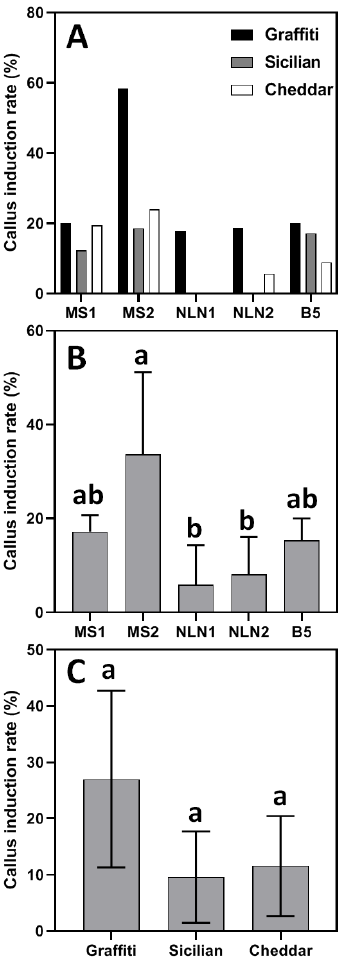
The five media formulations exhibited varying efficiency in callus induction (p<0.05). The MS2 medium (0.3 mg/L 2,4-D + 2.0 mg/L BAP) demonstrated exceptional efficacy, attaining 58.3% callus induction in Graffiti (Fig. 3 A) and an average of 33.6% callus induction across all genotypes (Fig. 3 B), significantly exceeding the performance of all other media, thereby validating its optimized hormonal equilibrium for microspore reprogramming. Conversely, NLN-based media (both hormonal combinations NLN1 and NLN2) failed to induce callus production in Sicilian (0% induction), whereas NLN1 did not stimulate callus formation in Cheddar, indicating genotype-specific incompatibility with the hormonal or nutritional makeup of NLN. Furthermore, NLN1 had the lowest callus induction results among all three genotypes, recording 5.93%. B5 medium demonstrated moderate yet consistent performance across all genotypes, with induction rates varying from 8.9% to 20.0%. These findings underscore the essential interplay between medium composition and genotype in cauliflower anther culture.
Genotype-specific callus induction
Among the selected genotypes, Graffiti exhibited exceptional responsiveness, with an average induction rate of 26.98%. On the other hand, the lowest induction rate was recorded in Sicilian at 9.6% (Fig. 3 C). Nonetheless, the disparities among the three genotypes were not statistically significant (p>0.05), suggesting that the culture medium and its interaction with genotype had a greater impact on callus formation than genotype alone. All genotypes notably commenced callus formation at 29–30 days (Data not shown), indicating comparable developmental schedule despite variations in efficiency.
Callus proliferation optimization
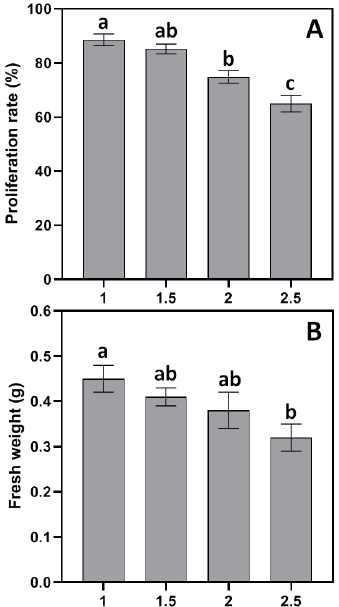
The efficiency of callus growth reached its maximum at 1 mg/L BAP (88.6%) and then decreased markedly to 74.8% and 65% at 2 mg/L and 2.5 mg/L BAP, respectively (Fig. 4 A). The fresh weight increase of the proliferating callus exhibited a same trend, with the 1 mg/L BAP medium yielding the largest weight gain (0.45 g) and the 2.5 mg/L BAP medium resulting in the lowest weight gain (0.3 g) (Fig. 4 B). Consequently, MS medium augmented with 1 mg/L BAP and 3 mg/L 2,4-D was identified as the optimal medium for callus growth among the evaluated formulations.
Discussion
The research revealed genotypic diversity in callus induction effectiveness, with Graffiti surpassing other genotypes. The efficacy of MS medium, especially with BAP and 2,4-D combinations, highlights the significance of cytokinin and auxin equilibrium in callus induction. On the other hand, the inadequate performance of NLN medium indicates its incompatibility with specific cauliflower genotypes. These findings underscore the necessity for genotype-specific refinement of anther culture procedures. The morphological variations in calli (Fig. 2) further emphasize genotype-media interactions. Graffiti generated friable, yellowish calli, signifying elevated embryogenic potential, whereas Sicilian and Cheddar developed compact calli, which may necessitate additional optimization for regeneration.
Notable genotype-specific and media-dependent reactions in cauliflower anther culture were observed. Of the three genotypes evaluated, Graffiti demonstrated the highest callus induction rate at 26.98%, followed by Cheddar at 11.58% and Sicilian at 9.60% (Fig. 3 C). This variation corresponds with prior observations in Brassica species, wherein genotype significantly influences androgenesis efficiency [31]. The MS2 medium (0.3 mg/L 2,4-D + 2.0 mg/L BAP) had the greatest callus induction rate, especially for Graffiti (58.3%). This outcome corroborates previous research highlighting the synergistic function of auxins (2,4-D) and cytokinins (BAP) in inducing cell proliferation and embryogenic callus development [21]. The exceptional performance of Graffiti indicates its genetic inclination towards enhanced totipotency, rendering it a viable choice for double haploid breeding initiatives. Notably, NLN medium, although often utilized in Brassica anther culture, exhibited suboptimal performance in this investigation, indicating that its effectiveness may be contingent upon genotype. In that context, Sicilian exhibited a negligible reaction in NLN media, with 0% callus formation in both NLN1 and NLN2, suggesting potential inhibitory interactions between its genetic composition and the hormonal constituents of NLN. This interaction represents an interesting study topic, since NLN callus induction rate in Graffiti genotype was somewhat normal (Fig. 3 A).
As for proliferation experiment, the combination of 1 mg/L BAP and 3 mg/L 2,4-D in MS medium resulted in the maximum callus proliferation rate (88.60%) and fresh weight (0.45 g) (Fig. 4). On the other hand, elevated BAP concentrations resulted in decreased efficiency. This pattern suggests that adequate BAP levels are essential; excessive cytokinin may interfere with cellular differentiation, as evidenced in other Brassica species [32].
The callus induction rate observed in Graffiti (26.98%) is markedly beyond the 5–15% range documented for other B. oleracea cultivars under comparable conditions [29], including those investigated in this study (Sicilian and Cheddar). This exceptional performance, along with the generation of friable embryogenic calli, indicates a genetic inclination towards androgenesis and improved stress response mechanisms during microspore reprogramming. Subsequent research should: (1) investigate NLN augmented with BAP (1–2 mg/L) to rehabilitate refractory genotypes such as Sicilian, and (2) utilize transcriptome analysis to discern biological markers linked to Graffiti’s elevated embryogenic potential. These measures will enhance genotype-specific protocols for the efficient production of doubled haploids in cauliflower breeding initiatives.
Conclusion
The experimental results position Graffiti as a superior genotype for cauliflower anther culture, achieving and average 26.98% callus induction efficiency. The optimized MS2 medium (0.3 mg/L 2,4-D + 2.0 mg/L BAP) demonstrated exceptional efficacy for callus induction, while the 1 mg/L BAP + 3 mg/L 2,4-D combination yielded unprecedented 88.6% proliferation rates, resolving long-standing challenges in hormonal balance for Brassica systems. Critical genotype-media interactions were evident in Graffiti’s consistent performance across media types compared to Sicilian’s complete failure on NLN formulations, underscoring the necessity of genotype-tailored protocols. Notably, Graffiti’s production of friable, embryogenic calli, distinct from the compact structures observed in other genotypes, provides breeders with valuable morphological markers for predicting regeneration potential.
By reducing doubled haploid production from 5–7 years to a single generation, this work offers transformative potential for hybrid breeding programs. Future efforts should prioritize transcriptomic analysis of Graffiti’s stress response pathways to identify molecular markers of androgenesis competence, alongside protocol adaptation for commercial F₁ hybrids and optimization of plantlet regeneration. These findings challenge the prevailing one-protocol-fits-all paradigm in Brassica tissue culture, advocating instead for customized solutions to accelerate genetic improvement in cauliflower and related crops.
Conflict of interest statement
The authors declared no conflict of interest.
Funding statement
The authors declared that no funding was received in relation to this manuscript.
Data availability statement
The authors declared that all used data are included in the manuscript.
References
- Singh S, Kalia P. Advances in Cauliflower (Brassica oleracea var. botrytis L.) Breeding, with Emphasis on India. Advances in Plant Breeding Strategies: Vegetable Crops. Cham: Springer International Publishing. 2021;247–301. DOI
- Mishra MG, Singh PK, Pandey P, Sohi MA. Breeding Approaches for Vegetable Crops. Golden Leaf PublishersTM: Uttar Pradesh, India. 2023.
- Singh BK, Singh B, Singh PM. Breeding cauliflower: A review. Int. J. Veg. Sci. 2018;24(1):58-84. DOI
- Kassa BA, Mekbib F, Assefa K. Effects of plant hormones and genotypes on anther culture response of safflower (Carthamus tinctorius L.). Sci. Afr. 2024;26:e02367. DOI
- Kumar KR, Singh KP, Bhatia R, Raju DV, Panwar S. Optimising protocol for successful development of haploids in marigold (Tagetes spp.) through in vitro androgenesis. Plant Cell Tissue Organ Cult. 2019;138(1):11-28. DOI
- Nandedkar K, Jha Z, Verulkar SB. Rice and Maize Haploids. In Doubled Haploids: Technological Advances and Role In Crop Improvement. Singapore: Springer Nature Singapore. 2025:159-95. DOI
- Zhou Y, Yang M, Zhao S, Shi H, Li Y, Gong W, Yang J, Wang J, Zou Q, Tao L, Kang Z. Rapid creation of interspecific hybrid progeny to broaden genetic distance through double haploid (DH) inducer in Brassica napus. Plants. 2022;11(5):695. DOI
- Jacquier NM, Gilles LM, Martinant JP, Rogowsky PM, Widiez T. Maize in planta haploid inducer lines: A cornerstone for doubled haploid technology. Doubled haploid technology: Volume 2: hot topics, Apiaceae, Brassicaceae, Solanaceae. 2021:25-48. DOI
- Kieffer ML. The in vitro manipulation of cauliflower (Brassica oleracea L. convar. botrytis (L.) Alef. var. botrytis L.) meristematic tissues for utilisation in genetic improvement programmes. Thesis, University of Plymouth. 1996.
- Hasan Y. Genetic dissection and QTL based modeling of vernalization and curd development in cauliflower (Brassica oleracea var. botrytis). Master thesis, Leibniz University Hannover. 2016.
- Toscano S, Trivellini A, Cocetta G, Bulgari R, Francini A, Romano D, Ferrante A. Effect of preharvest abiotic stresses on the accumulation of bioactive compounds in horticultural produce. Front. Plant Sci. 2019;10:1212. DOI
- Murata M, Orton TJ. Callus initiation and regeneration capacities in Brassica species. Plant Cell Tissue Organ Cult. 1987;11(2):111-23. DOI
- Dietert MF, Barron SA, Yoder OC. Effects of genotype on in vitro culture in the genus Brassica. Plant Sci. Lett. 1982;26(2-3):233-40. DOI
- Gerszberg A, Hnatuszko-Konka K, Kowalczyk T. In vitro regeneration of eight cultivars of Brassica oleracea var. capitata. In Vitro Cell Dev. Biol-Plant. 2015;51(1):80-7. DOI
- Siddiqi M. Integration of molecular tools with technological evaluation to monitor the performance of starter cultures used for yogurt production. Doctoral dissertation, University of Guelph. 2024.
- Siddiqi M. Characterizing bacterial diversity in undefined dairy starter cultures used for Cheddar cheese production. Doctoral dissertation, University of Guelph. 2020.
- Karlsen ST, Rau MH, Sánchez BJ, Jensen K, Zeidan AA. From genotype to phenotype: computational approaches for inferring microbial traits relevant to the food industry. FFEMS Microbiol. Rev. 2023;47(4):fuad030. DOI
- Sopory SK, Munshi M. Anther culture. In Vitro Haploid Production in Higher Plants: Volume 1-Fundamental Aspects and Methods. 1996:145-76. DOI
- Schaeffer GW. Role of microspores and anther culture in advancing technologies. Adv. Cell Cult. 1989;7:161-82. DOI
- Lichter R. Anther culture of Brassica napus in a liquid culture medium. Z. Pflanzenphysiol. 1981;103(3):229-37. DOI
- Sayem MA, Maniruzzaman M, Siddique SS, Al-Amin M. In vitro shoot regeneration through anther culture of Brassica spp. Bangladesh J. Agric. Res. 2010;35(2):331-41. DOI
- Zhang W, Fu Q, Dai X, Bao M. The culture of isolated microspores of ornamental kale (Brassica oleracea var. acephala) and the importance of genotype to embryo regeneration. Sci. Hortic. 2008;117(1):69-72. DOI
- Thaddi BN. Revolutionizing Plant Regeneration: Unleashing Somatic Embryogenesis in Sorghum bicolor (L.) Moench, via Immature Embryo and Mature Seed Explants. Research Square. 2023. DOI
- Monalisha R. Protocol development for in vitro regeneration of maize (Zea mays L.). Master Thesis, Birsa Agricultural University. 2017.
- Sahoo KK, Tripathi AK, Pareek A, Sopory SK, Singla-Pareek SL. An improved protocol for efficient transformation and regeneration of diverse indica rice cultivars. Plant Methods. 2011;7(1):49. DOI
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15(3). DOI
- Nitsch JP, Nitsch C. Haploid plants from pollen grains. Science. 1969;163(3862):85-7. DOI
- Gamborg OL, Miller R, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell. Res. 1968;50(1):151-8. DOI
- El Sharabasy SF. Effect of Vitamins (pyridoxine and nicotinic acid), Thiamine-Hcl and Myo-Inositol at Different Concentrations on Free Amino Acids and Indoles Content of Embryogeinic Callus of in vitro Date Oalm (Sakkoty and Bartamuda Cultivar). Mater. Res. Proc. 2019;11. DOI
- Tian H, Yao CY, Sun MX. High frequency conversion of microspore-derived embryos of Brassica napus cv. Topas by supplemental calcium and vitamins. Plant Cell Tissue Organ Cult. 2004;76(2):159-65. DOI
- Bhattacharya A, Palan BV, Mali K, Char B. Exploiting double haploidy in cauliflower (Brassica oleracea var. botrytis L.) for crop improvement. J. Appl. Hortic. 2017;19(2):101-5. DOI
- Ferrie AM, Möllers C. Haploids and doubled haploids in Brassica spp. for genetic and genomic research. Plant Cell Tissue Organ Cult. 2011;104(3):375-86. DOI
Cite this article:
Ahadi, M., Guo, R. Evaluation of anther culture and callus induction efficiency in three cauliflower genotypes using different media compositions. DYSONA – Applied Science, 2026;7(1): 20-27. doi: 10.30493/das.2025.530918

