Ammar Mostafa 1*; Questan Ameen 2
1, Department of Animal Production, Faculty of Agriculture Engineering, Latakia University, Latakia, Syria
2, Department of Animal Science, College of Agriculture Sciences, University of Sulaimani, Sulaymaniyah, Iraq
E-mail:
ammarmostafa110@gmail.com
Received: 23/09/2025
Acceptance: 23/10/2025
Available Online: 25/10/2025
Published: 01/01/2026

Manuscript link
http://dx.doi.org/10.30493/DAS.2025.548892
Abstract
The application of food by-products in poultry production has emerged as a compelling area of investigation in recent years. This research assessed the efficacy of pomegranate peel as a nutritional additive in broiler feed. To achieve this objective, a total of 120 unsexed one-day-old Ross 308 chicks, with an average initial body weight of 41.40 g, were randomly allocated into four experimental groups, each consisting of three replicates (10 birds per replicate). The T1 group, serving as the control, was administered a basal diet devoid of any supplementation. In contrast, groups T2, T3, and T4 were given the basal diet enhanced with pomegranate peel powder at concentrations of 0.1%, 0.3%, and 0.6%, respectively. Notable improvements in overall weight, weight gain, feed conversion efficiency, as well as protein, albumin, and globulin concentrations were recorded in T3 and T4 relative to the control group. Conversely, T3 and T4 treatments led to notable decreases in blood glucose levels, total cholesterol, and the activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). The addition of pomegranate peel exhibited a more pronounced effect at the 0.6% level (T4). Consequently, pomegranate peel can be effectively employed at the specified level as a cost-effective functional feed additive in broiler diets.
Keywords: Pomegranate peel, Broiler, Growth performance, Feed efficiency, Cholesterol
Introduction
Recent estimates indicate that approximately one-third of global food production is lost or wasted at different stages of the food supply chain, with significant variations in the extent of losses across regions and food categories. The potential losses in more perishable commodities, including fruits and vegetables, could be as high as 50%. As a result, one of the primary objectives set forth by the United Nations Sustainable Development Goals (SDGs) is to achieve a reduction in food waste by nearly 50% by the year 2050 [1]. Realizing this ambitious objective necessitates the adoption of the circular economy concept, which acts as a core tenet of sustainable innovation. The circular economy underscores the importance of establishing a society and economy devoid of waste, focusing on the reduction of losses and the enhancement of resource efficiency. The valorization of food waste involves the strategic use of these by-products as valuable resources for the creation of new products, rather than their immediate disposal. Significantly, food waste, alongside its uses in energy, agriculture, and animal feed, serves as a crucial raw material for the extraction of bioactive compounds, such as antioxidants and dietary fibers [2][3].
Pomegranate (Punica granatum L.) is among the most ancient fruits recognized globally and holds significant cultural importance in the Mediterranean region. This crop is widely grown across numerous nations, attributed to its various applications in regional culinary practices and its established health advantages [4]. Pomegranate is well-known for its significant levels of bioactive compounds, such as polyphenols, flavonoids, and tannins, which have been documented to demonstrate various biological effects, including antioxidant, antimicrobial, and anticancer properties. It is important to highlight that a significant proportion of these bioactive compounds is found in the peel, which is often discarded during processing. This practice results in considerable waste, estimated to exceed 3.6 million tons each year [5].
The incorporation of antibiotic growth promoters in poultry feed has generated significant apprehension owing to the rise of antibiotic-resistant bacterial strains and the buildup of drug residues in meat products. This phenomenon presents significant risks to poultry production and poses concerns for public health. As a result, contemporary developments in poultry production have increasingly focused on the integration of plant-based bioactive substances as natural feed supplements. In this context, pomegranate serves as an economical and sustainable resource for enhancing feed supplementation. Derivatives like pomegranate peel and seed powders and extracts exhibit significant nutritional value, various health-enhancing properties, and the potential for advantageous economic outcomes [6].
A number of investigations have explored the effects of integrating pomegranate peel powder into the diets of broiler chickens. For example, it has been observed that the enhancement of growth performance occurs with the supplementation of pomegranate peel powder at concentrations of 0.75% and 1% [7]. In a similar vein, significant improvements in growth performance, along with specific hematological and biochemical parameters, were observed with the incorporation of 0.5% pomegranate peel powder [8]. Consistent with these findings, the addition of varying concentrations of pomegranate peel powder (0.25%, 0.5%, 1%, and 1.5%) led to notable enhancements in all assessed characteristics, encompassing growth performance and blood biochemical parameters [9]. Conversely, the addition of 4% pomegranate peel powder demonstrated beneficial effects on the performance of broilers, while an inclusion level of 8% resulted in negative outcomes [10]. In a comparable manner, incorporating 2% pomegranate peel into the diet adversely influenced growth performance metrics in broilers [11]. Consequently, in light of the extensive evidence supporting the beneficial effects of pomegranate peel powder on growth and biological parameters in broilers, it is essential to precisely determine the optimal quantities of additives to incorporate into basal diets.
In light of the aforementioned considerations, additional research is essential to ascertain the ideal inclusion levels and to explore their biological potential. This study was conducted to assess the impact of dietary supplementation with pomegranate peel powder on the growth performance, hematological, and biochemical characteristics of Ross 308 broilers, while identifying the optimal inclusion level for practical use.
Materials and Methods
Preparation of pomegranate peel powder
Fresh pomegranate peels were separated from the arils and seeds. The peels were thoroughly washed under running water to eliminate impurities and were then shade-dried to maintain the integrity of heat- and light-sensitive bioactive compounds. Following this, the dried peels were ground with an electric grinder to produce a fine powder. The resulting powder was stored in airtight bags, protected from moisture, light, and heat, until it was needed for further applications.
Experimental design, management, feeding, and health program
The experiment utilized 120 one-day-old unsexed Ross 308 broiler chicks sourced from a private hatchery. Following the first body weight measurement of each chick, the birds were randomly allocated into four experimental groups, each including 30 birds, with three replicates per group (10 birds per replication). The trial spanned 42 days, from June 12 to July 23, 2025, and took place at a private farm in Tartous Governorate, Syria.
The experimental facility was established in accordance with stringent biosecurity protocols, which were upheld throughout the trial to guarantee avian health and safety of the used specimens and workers. A 5-cm layer of wood shavings served as bedding in a semi-enclosed enclosure measuring 15 m² with a height of 3 m, sustaining a stocking density of 10 birds/m². The temperature was first established at 32–33°C for the first two days and thereafter decreased by 2°C weekly until the conclusion of the trial, utilizing precision temperature and humidity control apparatus. Illumination was maintained continuously for 24 hours daily during the initial week and subsequently decreased to 22 hours per day until the subjects reached 42 days of age.
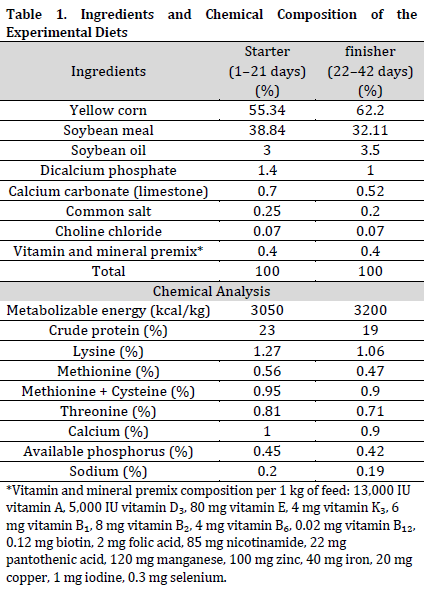
Birds were provided with balanced diets in two phases (starter/finisher), designed to fulfill the nutritional standards established by the National Research Council [12] (Table 1). Birds were randomly assigned to four feeding groups: the control group (T1) recieved the baseline feed without supplementation, whereas groups T2, T3, and T4 were provided the basal diet enriched with 0.1%, 0.3%, and 0.6% pomegranate peel powder, respectively. Diets were designed to include all critical nutrients, guaranteeing balanced energy, crude protein, vital amino acids, and minerals in accordance with the approved requirements for broilers.
A thorough health and vaccination initiative was executed. Access to the pens was limited to trained agricultural staff after daily sanitizing procedures. Vaccines were delivered through drinking water according to this schedule: Newcastle disease (Clone 30) on days 7, 21, and 32; Infectious Bronchitis (H120) on day 7; and Gumboro (Gumboro TM) on day 14. These techniques sought to safeguard birds from prevalent viral infections and guarantee consistent development performance during the trial.
Growth performance parameters
All chicks were individually weighed in each replicate upon arrival at the farm, and the average body weight was recorded weekly, as well as on days 21 and 42. Prior to weighing, birds were fasted for three hours to ensure accurate measurements. A precision electronic scale with a capacity of up to 5,000 g was used for this purpose.
Production performance parameters were calculated using the following equations:
Weight Gain (g) = Body weight at the end of the period (g) – Body weight at the beginning of the period (g)
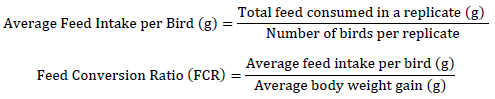
Blood biochemical parameters
Blood samples were collected from the birds following the guidelines for blood sampling in poultry [13] at the end of the trial (day 42), using 3-mL syringes from the wing vein of nine birds per group (three birds per replicate). After collection, samples were transferred into sterile Vacutainer tubes without anticoagulant (EDTA-free) to facilitate biochemical analyses, including total protein, albumin, globulin, glucose, total cholesterol, as well as aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzyme activities. The tubes were positioned at an angle to promote serum separation and transported to the laboratory in an ice-cooled container. For serum preparation, samples were centrifuged at 3,500 rpm for 5 minutes to obtain clear serum, which was then stored in Eppendorf tubes at -15 to -20°C until analysis.
Total protein: Measured using the Biuret method with a commercial kit (BioSystems®, Spain), with absorbance read at 545 nm on a spectrophotometer [14].
Albumin: Determined using the bromocresol green method with a commercial kit (BioSystems®, Spain), read at 630 nm [15].
Globulin: Calculated by subtracting albumin from total protein [16].
Total cholesterol and glucose: Assayed enzymatically using commercial kits (BioSystems®, Spain), with readings at 500 nm for cholesterol and 505 nm for glucose [17].
AST and ALT activities: Measured calorimetrically using commercial kits (BioSystems®, Spain), with absorbance read at 340 nm [18].
Statistical analysis
Data were analyzed using SPSS software version 29. One-way analysis of variance (ANOVA) was performed to compare the experimental groups arranged in a completely randomized design. Significant differences between means were further assessed using Duncan’s multiple range test at a significant level of 0.05 [19].
Results
Growth performance parameters
At 21st day mark, no significant differences in body weight were recorded between T1 (882.63±6.82 g) and T2 (883.51±6.83 g) (Table 2), however both were significantly lower than T4 (911.75±7.05 g). By Day 42, a comparable pattern was noted, with T4 displaying the highest body weight (2783.34±10.50 g), significantly surpassing all other groups. T1 (2694.42±10.17 g) and T2 (2697.11±10.18 g) did not differ substantially from each other.
The patterns of body weight gain corresponded with the final body weight. From Days 1 to 21, T4 (870.33±6.67 g) exhibited considerably superior weight increase compared to T1 and T2. Between Days 22 and 42, T4 (1871.58±3.46 g) exhibited a marked advantage, achieving considerably greater gains than T1, T2, and T3. Throughout the 42-day duration, T4 exhibited the largest total weight gain (2741.91±10.12 g), greatly surpassing other treatments.
Elevated feed intakes were recorded in T1 and T2 throughout the experiment in comparison to T3 and T4; however, no significant differences in term of feed intake were observed between the different treatments and control. In contrast, feed conversion ratio (FCR) shown considerable enhancement with the addition of pomegranate peel. In the period from Days 1 to 21, the Feed Conversion Ratio (FCR) was comparable between T1 (1.36±0.006) and T2 (1.36±0.004), but significantly reduced (improved) in T3 (1.33±0.003) and T4 (1.30±0.002). Elevated FCR values were noted between days 22 and 42, with T4 exhibiting a much superior (lower) FCR compared to alternative therapies. It can be deduced that the inclusion of pomegranate peel powder, especially at the 0.6% concentration (T4), markedly improved body weight, weight gain, and feed conversion ratio relative to the control group, suggesting enhanced growth performance and nutrient efficiency in broiler chickens (Table 2).
Blood biochemical parameters
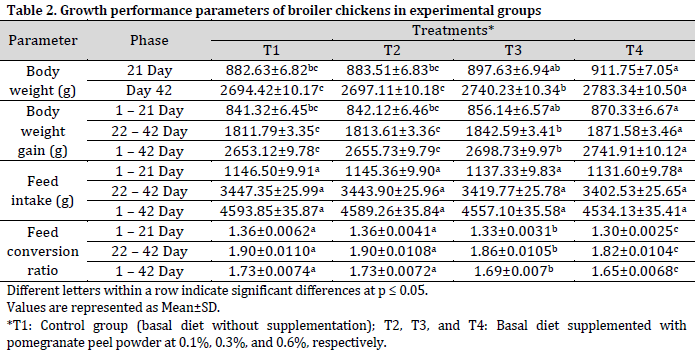
The concentrations of total protein, albumin, and globulin exhibited a significant increase in correlation with elevated levels of pomegranate peel inclusion. The specimens in the T4 group demonstrated the highest levels of total protein (3.70±0.029 g/dl) and albumin (1.62±0.019 g/dl). These values were significantly higher compared to the control group (T1), which recorded total protein levels of 3.50±0.019 g/dl and albumin levels of 1.53±0.014 g/dl. Globulin exhibited a comparable trend, with T4 (2.09±0.023 g/dl) significantly surpassing T1 (1.97±0.016 g/dl). T3 (0.3%) exhibited markedly higher values across all three protein fractions in comparison to T1.
Serum glucose levels were markedly elevated in the T1 and T2 groups when contrasted with the T3 and T4 groups. The control group (T1) exhibited the highest cholesterol level (168.44±1.50 mg/dl), which was significantly elevated compared to both T3 (163.73±1.46 mg/dl) and T4 (158.67±1.42 mg/dl).
Indicators of hepatic integrity (represented by AST and ALT activity) exhibited a notable reduction in the supplemented cohorts, especially in the T4 group. The activity of AST in T4 (186.97±1.31 U/L) exhibited a significant reduction when compared to T1 (194.35±1.36 U/L), while T3 (189.50±1.33 U/L) demonstrated a notable improvement as well. In a comparable manner, ALT levels were observed to be at their minimum in T4 (19.00±0.104 U/L), which was significantly lower than T1 (19.53±0.113 U/L). Additionally, T3 (19.18±0.109 U/L) demonstrated a significant improvement over the control group.
Discussion
The inclusion of different concentrations of pomegranate peel in broiler diets demonstrated a beneficial impact on both production performance and blood biochemical indicators. The inclusion level of 0.6% yielded significant enhancements in growth performance metrics, which encompassed increases in body weight and average weight gain, a marginal decrease in feed intake, and a favorable adjustment in the feed conversion ratio (FCR). Simultaneously, there was a notable enhancement in blood biochemical parameters, characterized by increased serum protein levels and decreased concentrations of glucose, total cholesterol, as well as reduced activities of AST and ALT.
In alignment with earlier research, the addition of 0.75% and 1% pomegranate peel powder enhanced feed efficiency by promoting body weight gain while simultaneously decreasing feed intake [7]. In a similar vein, the incorporation of 0.5% pomegranate peel powder resulted in an increase in body weight without notable alterations in feed intake, while also leading to a significant reduction in feed conversion ratio; however, there were non-significant increases observed in total protein and glucose levels, accompanied by significant decreases in AST, ALT, and total cholesterol levels [8].
In other studies, the incorporation of varying concentrations of pomegranate peel powder (0.25%, 0.5%, 1%, and 1.5%) resulted in notable enhancements in body weight, weight gain, feed intake, and feed conversion ratio (FCR). This was also associated with significant reductions in AST and ALT enzyme activities, alongside increases in total protein, albumin, and globulin levels [9]. In contrast, there were significant increases in body weight; however, the reductions in feed conversion ratio were not statistically significant. Additionally, no notable changes were detected in glucose, total protein, or albumin levels, while total cholesterol exhibited a significant decrease at inclusion levels of 0.25%, 0.5%, and 1% [20].
At elevated inclusion levels, the addition of 8% pomegranate peel powder resulted in negative impacts on growth performance and blood biochemical parameters. Similarly, at the 4% inclusion level, feed efficiency did not show significant improvement, even though there were reductions in glucose and cholesterol levels, along with a decrease in serum protein at this concentration [10]. Moreover, the incorporation of 2% pomegranate peel resulted in adverse effects on growth performance and serum protein levels, leading to notable decreases in AST, ALT, cholesterol, and glucose concentrations [11].
The enhancement observed in growth performance metrics can be ascribed to the abundant presence of dietary fibers, vitamins, and bioactive chemical constituents in pomegranate peel, including polyphenols, gallic acid, ellagic acid, and punicalagin, which exhibit antioxidant, anti-inflammatory, and antimicrobial characteristics [21]. Research indicates that phenolic acids play a significant role in reducing oxidative stress. Specifically, protocatechuic acid (PCA) has been found to enhance the activity of intracellular antioxidant enzymes, including superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px). This enhancement promotes the scavenging of reactive oxygen species (ROS) and contributes to the alleviation of oxidative stress [22][23].
Furthermore, numerous polyphenolic compounds demonstrate inhibitory actions against a range of microbial pathogens by interfering with bacterial membranes via protein precipitation and inhibiting enzymes like glycosyltransferases, resulting in microbial cell lysis [24][25]. A separate investigation revealed the antimicrobial properties of pomegranate peel against pathogenic bacteria such as Escherichia coli. This effect was observed alongside the preservation of a beneficial gut microbiota composition, an increase in villus height in relation to the depth of the small intestinal lumen, and a subsequent enhancement in growth performance in broilers [26]. Consequently, pomegranate peel provides feed enriched with nutritionally and biologically significant compounds, alleviates oxidative stress and intestinal inflammation, and promotes gut health by augmenting the absorptive surface area and sustaining a balanced microbiome. This leads to enhanced nutrient absorption with reduced feed consumption, resulting in greater body weight and a more favorable feed conversion ratio.
The observed increases in total protein, albumin, and globulin levels, in addition to the notable decrease in AST and ALT activity suggest an enhancement in liver function and an improvement in the efficiency of protein synthesis. The protective effects on the liver are mainly linked to the polysaccharides found in pomegranate peel, which demonstrate significant activity in scavenging free radicals [27]. Research indicates that these compounds can increase albumin and total protein levels [28], while also improving humoral immunity in avian species. Polyphenols are known to influence phagocytic immune responses and stimulate B lymphocytes, leading to their differentiation into plasma cells that produce immunoglobulins [29][30].
The mechanisms underlying the hypoglycemic and anti-hyperglycemic properties of pomegranate peel are attributed to the inhibition of α-amylase and α-glucosidase enzymes. The inhibition of these enzymes results in a postponement of carbohydrate digestion, consequently leading to a diminished rate of glucose release into the bloodstream [31]. A recent investigation identified the interaction of punicalagin, sourced from pomegranate peel, as a contributing factor to the delayed metabolism of carbohydrates, specifically through its effect on α-glucosidase [32].
The cholesterol-lowering effect can be ascribed to the high concentration of phenolic compounds, including ellagitannins and their derivatives, found in pomegranate peel, which demonstrate various bioactive properties. Urolithins have been shown to influence cholesterol metabolism through the reduction of Coriobacteriaceae bacteria and modifications in bile acid pools. This process enhances fat absorption in the intestine and offers protective benefits for cardiovascular health [33].
The noted decrease in liver enzymes (AST and ALT) correlates with the hepatoprotective characteristics of compounds found in pomegranate peel. Polysaccharides and flavonoids, including quercetin, demonstrate the ability to scavenge free radicals, thereby reducing oxidative stress markers in serum, such as MDA and ROS. Furthermore, they enhance the activities of antioxidant enzymes like SOD, CAT, and GSH, contributing to effective liver protection [27][34]. Several investigations have indicated a notable reduction in AST and ALT levels subsequent to supplementation [28].
Overall, the inclusion of pomegranate peel powder, particularly at a concentration of 0.6% (T4), demonstrated a significant association with increased protein synthesis, enhanced lipid metabolism characterized by reduced cholesterol levels, and improved liver function indicated by lower AST and ALT values in broilers at the conclusion of the 42-day trial. The biochemical enhancements correspond with the previously observed advancements in growth performance and feed efficiency.
5. Conclusions
The present study demonstrated that dietary supplementation with pomegranate peel powder, specifically at 0.6% (T4), markedly improved growth performance in Ross 308 broiler chickens, as indicated by increased body weight, elevated weight gain, and enhanced feed conversion ratio relative to the control group. Despite no significant differences in feed intake among treatments, T4 exhibited enhanced nutrient utilization efficiency. Furthermore, notable enhancements in blood biochemical profiles have been observed, including elevated levels of total protein, albumin, and globulin, alongside decreased glucose, cholesterol, and liver enzyme activity (AST and ALT). The results demonstrate that pomegranate peel powder, particularly at a concentration of 0.6%, enhances growth and metabolic health in broiler chickens.
Conflict of interest statement
The authors declared no conflict of interest.
Funding statement
The authors declared that no funding was received in relation to this manuscript.
Data availability statement
The authors declared that all experimental data will be available upon reasonable request from the corresponding author.
References
- FAO. Technical platform on the measurement and reduction of food loss and waste. Rome: Food and Agriculture Organization of the United Nations. 2022. [Accessed in 2025 Sep 22]. Available from: Link
- Girotto F, Alibardi L, Cossu R. Food waste generation and industrial uses: A review. Waste Manag. 2015;45:32-41. DOI
- Grillo G, Boffa L, Talarico S, Solarino R, Binello A, Cavaglià G, Bensaid S, Telysheva G, Cravotto G. Batch and Flow Ultrasound-Assisted Extraction of Grape Stalks: Process Intensification Design up to a Multi-Kilo Scale. Antioxidants. 2020;9(8):730. DOI
- Singh B, Singh JP, Kaur A, Singh N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018;261:75-86. DOI
- Grillo G, Capaldi G, Radošević K, Jakopović Ž, Markov K, Brnčić M, Gallina L, Calcio Gaudino E, Cravotto G. Unlocking the bioactive potential of pomegranate peels: A green extraction approach. Antioxidants. 2023;12(10):1796. DOI
- Abd El-Ghany WA. A Natural Feed Additive Phytobiotic, Pomegranate (Punica granatum L.), and the Health Status of Poultry. Maced. Vet. Rev. 2023;46(2):113-28. DOI
- Ahmadipour B, Pat S, Khajali F. The protective effect of pomegranate peel powder on pulmonary hypertension in broiler chickens. JSM Biomark. 2018;4(1):1013.
- Choudhari BL, Shende KA, Meel MS, Joshi M, Chavhan DM. Effect of Pomegranate (Punica granatum) Peel Powder and Eucalyptus (Eucalyptus globulus) Leaves Powder on Growth Performance and Haemato-biochemical Study on Broilers. Int. J. Bio-resour. Stress Manag. 2025;16(6):1-6. DOI
- Elnaggar A, Elsebai A, Eltahawy W, Elattar F. Effect of Dietary Inclusion of Pomergranate Peel Powder (Punica grantum) on Growth Performance and Some Physiological Parameters of Broiler Chicks. J. Agric. Environm. Sci. 2022;21(2):19-44. DOI
- Ghasemi-Sadabadi M, Ebrahimnezhad Y, Maheri-Sis N, Ghalehkandi JG, Shaddel-Teli A. Immune response and antioxidant status of broilers as influenced by oxidized vegetable oil and pomegranate peel. J. Anim. Sci. Technol. 2021;63(5):1034-63. DOI
- Younis M, Abdo SG, Elmakarem MAA, Mustafa FEA, Fawaz MA. Evaluating dried pomegranate peel as a functional feed additive: effects on growth, carcass traits, and gut health in broilers. Trop. Anim. Health Prod. 2025;57(4):221. DOI
- National Research Council (NRC). Nutrient requirements of poultry: Nutrient requirements of domestic animals. 9th rev. ed. Washington (DC): National Academy Press; 1994:176. Available from: Link
- Morishita TY. Poultry blood collection (Phr Factsheet). Ohio State University Extension and Western University of Health Sciences. 2019.
- Zaia DAM, Marques FR, Zaia CTBV. Spectrophotometric determination of total proteins in blood plasma: a comparative study among dye-binding methods. Braz. Arch. Biol. Technol. 2005;48(3):385-8. DOI
- Schmidt EMDS, Paulillo AC, Locatelli-Dittrich R, Beltrame OC, de Oliveira EG. Comparison of different methods of measuring albumin concentration in ring-necked pheasants. Comp. Clin. Pathol. 2012;22(2):261-2. DOI
- Rifai N. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics-E-Book. Elsevier Health Sciences. 2017.
- Torres-Gamez J, Rodriguez JA, Paez-Hernandez ME, Galan-Vidal CA. Application of Multivariate Statistical Analysis to Simultaneous Spectrophotometric Enzymatic Determination of Glucose and Cholesterol in Serum Samples. Int. J. Anal. Chem. 2019;2019:1-5. DOI
- Huang X, Choi Y, Im H, Yarimaga O, Yoon E, Kim H. Aspartate Aminotransferase (AST/GOT) and Alanine Aminotransferase (ALT/GPT) Detection Techniques. Sensors. 2006;6(7):756-82. DOI
- George D, Mallery P. IBM SPSS statistics 29 step by step: A simple guide and reference. Routledge; 2024.
- Gosai AS, Pawar MM, Patil SS, Ankuya KJ, Srivastava AK, Ashwar BK. Effect of pomegranate (Punica granatum) peel powder supplementation on performance, carcass characteristics and haemato-biochemical parameters of broiler chickens. Indian J. Anim. Sci. 2023;93(5):481-6.
- Singh J, Kaur HP, Verma A, Chahal AS, Jajoria K, Rasane P, Kaur S, Kaur J, Gunjal M, Ercisli S. Pomegranate Peel Phytochemistry, Pharmacological Properties, Methods of Extraction, and Its Application: A Comprehensive Review. ACS Omega. 2023;8(39):35452-69. DOI
- Cadena-Iñiguez J, Santiago-Osorio E, Sánchez-Flores N, Salazar-Aguilar S, Soto-Hernández RM, Riviello-Flores MDLL, Macías-Zaragoza VM, Aguiñiga-Sánchez I. The Cancer-Protective Potential of Protocatechuic Acid: A Narrative Review. Molecules. 2024;29(7):1439. DOI
- Laskar YB, Mazumder PB. Insight into the molecular evidence supporting the remarkable chemotherapeutic potential of Hibiscus sabdariffa L. Biomed. Pharmacother. 2020;127:110153. DOI
- Skenderidis P, Mitsagga C, Giavasis I, Petrotos K, Lampakis D, Leontopoulos S, Hadjichristodoulou C, Tsakalof A. The in vitro antimicrobial activity assessment of ultrasound assisted Lycium barbarum fruit extracts and pomegranate fruit peels. J. Food Meas. Charact. 2019;13(3):2017-31. DOI
- Kupnik K, Leitgeb M, Primožič M, Postružnik V, Kotnik P, Kučuk N, Knez Ž, Marevci MK. Supercritical Fluid and Conventional Extractions of High Value-Added Compounds from Pomegranate Peels Waste: Production, Quantification and Antimicrobial Activity of Bioactive Constituents. Plants. 2022;11(7):928. DOI
- Xu P, Wang J, Chen P, Ding H, Wang X, Li S, Fan X, Zhou Z, Shi D, Li Z. Effects of pomegranate (Punica granatum L.) peel on the growth performance and intestinal microbiota of broilers challenged with Escherichia coli. Poult. Sci. 2024;103(2):103304. DOI
- Zhai X, Zhu C, Zhang Y, Sun J, Alim A, Yang X. Chemical characteristics, antioxidant capacities and hepatoprotection of polysaccharides from pomegranate peel. Carbohydr. Polym. 2018;202:461-9. DOI
- Abd El Fattah S, M.A. Omar A, Abd El Ghani E, Keshta A. Effect of N-Acetyl cysteine and Pomegranate Peel Water Extract on Hepatotoxicity Induced by Paracetamol. Biochem. Lett. 2018;13(1):14-29. DOI
- Shakoor H, Feehan J, Apostolopoulos V, Platat C, Al Dhaheri AS, Ali HI, Ismail LC, Bosevski M, Stojanovska L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients. 2021;13(3):728. DOI
- Hachimura S, Totsuka M, Hosono A. Immunomodulation by food: impact on gut immunity and immune cell function. Biosci. Biotechnol. Biochem. 2018;82(4):584-99. DOI
- Hasan AM, Redha AA, Mandeel Q. Phytochemical determinations of pomegranate (Punica granatum) rind and aril extracts and their antioxidant, antidiabetic and antibacterial activity. Nat Prod Chem Res. 2018;6(4):1-9. DOI: DOI
- Liu Y, Kong KW, Wu D, Liu H, Li H, Zhang J, Gan R. Pomegranate peel-derived punicalagin: Ultrasonic-assisted extraction, purification, and its α-glucosidase inhibitory mechanism. Food Chem. 2022;374:131635. DOI
- Cortés-Martín A, Iglesias-Aguirre CE, Marín A, Romo-Vaquero M, Vallejo F, Espín JC, Victoria Selma M. Urolithin A production drives the effects of pomegranate on the gut microbial metabolism of bile acids and cholesterol in mild dyslipidaemic overweight and obese individuals. Food Funct. 2024;15(5):2422-32. DOI
- Murtaza S, Khan JA, Aslam B, Faisal MN. Pomegranate peel extract and quercetin possess antioxidant and hepatoprotective activity against concanavalin A-induced liver injury in mice. Pak. Vet. J. 2021;41(2).
Cite this article:
Mostafa, A., Ameen, Q. Pomegranate peel as a functional feed additive in broiler diets: effects on growth performance, feed efficiency, and blood biochemistry. DYSONA – Applied Science, 2026; 7(1): 110-118. doi: 10.30493/das.2025.548892

