Sneha Gupta1; Abdullah Sherikar1; Aman Upaganlawar1*; Chandrashekhar Upasani1
1, Department of Pharmacology, SNJBs SSDJ College of Pharmacy, Savitribai Phule Pune University, Pune, India
E-mail:
amanrxy@gmail.com
Received: 15/08/2019
Acceptance: 18/09/2020
Available Online: 21/09/2020
Published: 01/10/2020

Manuscript link
http://dx.doi.org/10.30493/DLS.2020.243982
Abstract
The present pre-clinical activity was undertaken to screen the two antioxidants, mainly α-lipoic acid and ferulic acid alone, and in combination in neuropathic pain induced by diabetes in rats. The activity was confirmed by assessing various behavioral as well as biochemical and histopathological studies. The study was performed on adult albino rats. The rats were divided into different groups, and each group contained six rats. Diabetic neuropathy in rats was induced by administering a freshly prepared single dose of streptozotocin (60 mg/kg, i.p). After the development of neuropathy, three groups were treated with α-lipoic acid (25 mg/kg/day, p.o), ferulic acid (10 mg/kg/day, p.o), or the standard drug Pregabalin (30 mg/kg/day i.p). The final group received a combination of antioxidants, (α-lipoic acid and ferulic acid) at 12 and 5 mg/kg, p.o, respectively, for two weeks. Neuropathic pain was assessed using mechanical allodynia, mechanical hyperalgesia, cold allodynia, and thermal allodynia. Biochemical parameters of blood glucose, nitric oxide, level of lipid peroxidase, reduced glutathione, and membrane-bound ATPase activities were also studied. Neuropathic pain-induced rats showed a significant alteration in behavioral and biochemical parameters. Treatment with α-lipoic acid in combination with Ferulic acid significantly restored the altered parameters towards normal as compared to single antioxidants, thus providing proper neuroprotection. This effect might be due to the strong free radical scavenging potential of α-lipoic acid and ferulic acid.
Keywords: Neuropathic pain, α-lipoic acid, Ferulic acid, Oxidative stress, Streptozotocin
Abbreviations: α-lipoic acid (ALA), Streptozotocin (STZ), Ferulic acid (FA), Pregabalin (PG), Intraperitoneal injection (i.p.), By mouth (p.o.), Diabetic neuropathy (DN), Neuropathic Pain (NP), Percentage of maximum possible effect (%MPE), Lipid peroxidation (LPO), Reduced glutathione (GSH), Nitric oxide level (NO)
Introduction
Diabetic neuropathy is considered one of the microvascular complications of long-term hyperglycemia. It has been reported that people with chronic diabetes mellitus are more prone to the development of neuropathy, which affects the quality of life of up to 60% of patients [1]. STZ-induced diabetes is a well-established animal model, which reports the involvement of oxidative stress in neuropathy.
Several studies have reported the preventive role of antioxidants in the progression of neuropathic pain [2]. α-lipoic acid (ALA), also known as thioctic acid, and its reduced dithiol form, i.e.,dihydrolipoic acid (DHLA), are known for their antioxidant activities [3][4]. ALA is reported to possess various pharmacological activities in animals, such as participating in the regeneration of exogenous and endogenous antioxidants, reparation of oxidized proteins, chelation of metal ions, and quenching of reactive oxygen species [5-9]. Ferulic acid (FA) is a polyphenol compound with potent antioxidant and anti-diabetic activities [10]. On the other hand, pregabalin is a well-known anti-diabetic drug and is reported as an effective agent for treating pain associated with various conditions such as diabetic neuropathy, post-herpetic neuralgia, and central neuropathic pain [11][12]. Combinations of antioxidants are reported to show synergistic activity [13-16]. Based on the available literature, this study was designed to investigate the combined effects of ALA and FA in streptozotocin-induced neuropathic pain in experimental animals by evaluating various behavioral and biochemical parameters.
Material and Methods
Animals
In the present study, albino rats of the Wister strain were used. The rats were procured from (LACSMI Biofarm, Aundh, Pune, Maharashtra, India). All the rats were housed separately in polypropylene cages with paddy husk as bedding material. The rats were maintained in a standard environment based on the guidelines provided by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. During the experiment, the rats had free access to water and standard laboratory food with proper nutrition value (Nutrivet Lab, Pune, India). The experimental protocols were reviewed and approved by the Institutional Animal Ethics Committee (IAEC) of the college with approval number SSDJ/ IAEC/2018-19/03.
Drugs and reagents
α-lipoic acid (ALA) and streptozotocin (STZ) were procured from Sigma Aldrich, USA. Ferulic acid (FA) was obtained from (Otto Kemmi PVT Ltd. Mumbai, India). Pregabalin (PG) was a gift sample from Sun Pharmaceuticals, Vadodara, Gujarat. Other chemicals used in the study were obtained from a standard supplier.
Preparation of drug solutions
All drug solutions were freshly prepared at the time of administration. STZ was dissolved in an ice-chilled freshly prepared citrate buffer (0.1M, pH 5.5). Arachis oil was used as a vehicle for ALA, whereas FA and PG were dissolved in 0.9% normal saline.
Induction and assessment of diabetes neuropathy
Diabetes neuropathy was induced in rats by administration of a single dose (60 mg/kg, i.p) of STZ. It was confirmed by estimating of 72hours blood glucose level using a Glucometer. Rats with blood glucose levels higher than 250mg/dl were considered diabetic and used for the development of neuropathic pain [17].
Experimental protocol
The day on which DN was developed is considered a basal reading (4 weeks). Since then, the treatment was carried out for two weeks, so the protocol was six weeks in total. The rats were divided into six groups
Rats in group I were assigned as a control group (CN), while all the other groups were injected with STZ. Group II rats were considered a Diabetic neuropathy (DN) group and received no further treatments. Rats in group III received PG as a standard neuroprotective drug (30mg/kg i.p. once a day for two weeks). Group IV and V rats received ALA (25mg/kg, p.o. once a day for two weeks) or FA (10mg/kg, p.o. once a day for two weeks), respectively. Group VI received both FA (12mg/kg, p.o.) and ALA (5mg/kg) once a day for two weeks.
Blood glucose level was estimated using a Glucometer (Nipro Diagnostics) by the end of the experiment. Food consumption was measured in terms of (g/day), water consumption was measured in terms of (ml/day), and body weight was measured in terms of (g) on the first week, basal week (4th week), and the final week (6th week).
Assessment of neuropathic pain
Assessment of NP was carried out using various behavioral methods:
- Mechanical allodynia using von Frey hair test [18]
- Mechanical hyperalgesia using Pinprick Test [19]
- Randall-Selitto analgesiometer [20]
- Thermal allodynia and hyperalgesia using Eddy’s hot plate method [21]
- Cold allodynia using acetone [22]
Assessment of biochemical parameters
At the end of the treatment period, rats were anesthetized using a high dose of thiopental sodium. Blood glucose levels were determined. The sciatic nerve was isolated, and a homogenate was made using ice-cold Tris hydrochloric buffered saline (10 mM, pH 7.4). After centrifugation, the supernatant was used for the estimation of various biochemical parameters. The cleared supernatant was used for the determination of lipid peroxidation (LPO) [23], reduced glutathione (GSH) [23], and nitric oxide level (NO) [24], and the sediment was used for the determination of ATPases.Na+/K+-ATPase [25], Ca+2ATPase [26] and Mg+2-ATPase [27].
Statistical analysis
The results were analyzed using Graph Pad Prism, version 0.5. One-way ANOVA followed by Dunnet ‘t’ test was applied in order to compare test groups for significant differences. The control group was compared with the diabetic neuropathy group ap<0.05, the Diabetic neuropathy group was compared with treatments bp<0.05, While the group treated with a combination of antioxidants was compared with ALA-treated xp<0.05, and FA-treated groups yp<0.05.
Results
Blood glucose level, body weight, food, and water intake
By the end of the experiment, blood glucose levels in all STZ groups (Group II-VI) were significantly higher than that of CN group. However, treatment with ALA+FA for two weeks displayed a significant reduction in blood glucose levels when compared with PG, DN, ALA, and FA-treated groups. Bodyweight was reduced in all diabetic groups, whereas food and water intake were increased compared to CN group. However, treatment PG, ALA, FA, or ALA+FA combination showed significant improvement in body weight, food intake, and water intake as compared to DN (Table 1).
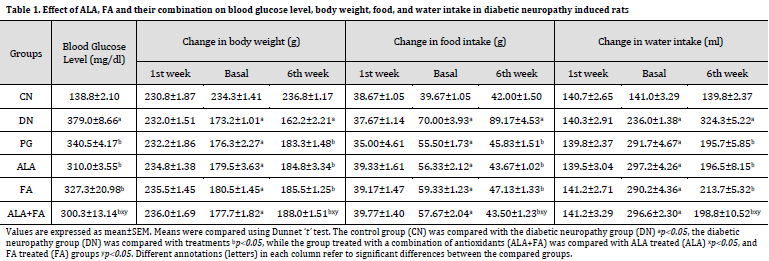
Behavioral changes
Mechanical allodynia was performed using von Frey Hair Test. It was found that the 50% threshold for paw withdrawal in DN rats was significantly (p<0.05) less compared to CN rats on basal evaluation (4th week), 5th and 6th week. Treatment with ALA+FA group showed a significant increase in the 50% threshold for paw withdrawal compared to individual antioxidants as shown in (Fig. 1 A).
Mechanical hyperalgesia was performed using the Randall-Selitto test. It was observed that the paw withdrawal latency in DN group was significantly (p<0.05) reduced as compared to CN group. ALA+FA-treated rats displayed a significant rise in paw withdrawal latency compared to DN, ALA, FA, and PG, as shown in (Fig. 1 B).
In Pinprick test, the paw withdrawal latency in DN rats was significantly (p<0.05) less compared to CN rats. Treatment with ALA+FA showed a significant rise in paw withdrawal latency as compared to DN, ALA, FA alone group (Fig. 1 C).
Cold allodynia was performed using the acetone method. it was found that paw withdrawal latency in DN rats was significantly (p<0.05) less than that of CN rats. The ALA+FA rats showed significant elevation in paw withdrawal latency compared to DN, ALA, and FA, as shown in (Fig. 1 D).
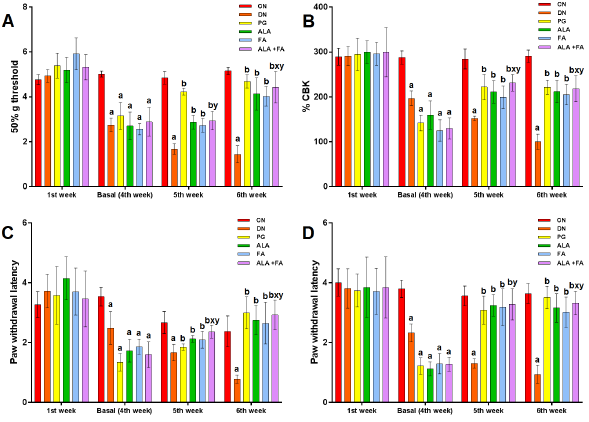
Eddy’s Hot Plate Test was performed to check the response to thermal hyperalgesia, and it was expressed in % Maximal possible effect (%MPE). %MPE in DN group showed a significant (p<0.05) reduction compared to CN animals. Rats treated with ALA and FA together or separately showed a significant rise in %MPE as compared to DN and ALA alone, as shown in (Fig. 2).
Endogenous antioxidants
The endogenous antioxidant markers such as LPO, GSH, and NO were measured in sciatic nerve homogenates. LPO level significantly increased (p<0.05), whereas the level of GSH was reduced considerably in DN rats compared to CN rats (Table 2). Treatment with ALA, FA, PG, and ALA+FA for two weeks showed a significant (p<0.05) reduction in the level of LPO, and a significant (p<0.05) rise in GSH level as compared to DN (Table 2). NO level was significantly (p<0.05) higher in DN group as compared to CN. Combined treatment of ALA+FA showed a significant (p<0.05) reduction in NO level compared to DN rats (Table 2).
Membrane-bound enzymes
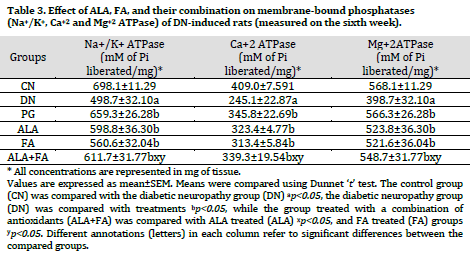
The activity of membrane-bound phosphatases such as Na+/K+, Ca+2, and Mg+2ATPase was found to be significantly (p<0.05) reduced in DN group compared to the control group. Treatment with ALA, FA, and PG for two weeks showed significant (p<0.05) increased Na+/K+, Ca+2, and Mg+2ATPase levels compared to the non-treated DN group. Combined treatment with ALA and FA showed further improvement in the level of membrane-bound phosphatases compared to other treated groups (Table 3).
Discussion
The present study was conducted to investigate the potential beneficial effects of two well-known antioxidants ALA and FA, in streptozotocin-induced diabetic neuropathy. ALA therapeutic effectiveness was previously reported in a broad spectrum of diseases such as heavy metal poisoning, ischemia-reperfusion injury, cardiovascular disorders, and neurodegenerative disease, which is probably due to its substantial antioxidant properties [4][28][29]. Additionally, FA is used as an antioxidant in the management of diabetes, aging, and cancer, and is also used as a pulmonary protecting agent [30]. PG is a novel antiepileptic agent that primarily binds to the alpha-2-delta subunit of voltage-gated Ca2+ channels and exerts analgesic action [31].
The current study showed that the administration of ALA and FA reduced the elevated level of glucose. This observation was in line with previous reports [28][32]. FA was reported to stimulate insulin secretion and increase the utilization of glucose by extrahepatic tissues [27][33]. Rats with DN showed increased food and water consumption, which corresponds to the previously reported study [34]. The treatment with ALA and FA alone and their combination significantly reduced the food and water intake. Rats with DN showed decreased body weight compared to the control group, similar to the results reported by[35]. A decrease in body weight after STZ administration might be due to the degradation of structural proteins, which increases muscle wasting [31][38]. However, the combined ALA + FA treatment enhanced body weight significantly.
DN is associated with a decrease in paw withdrawal latency, which can be assessed by various behavioral nociceptive tests. Using the von Frey hair test, it was found that STZ induced a reduction in the 50 % paw withdrawal threshold, and the treatment with ALA and FA with their combination significantly elevated the 50% threshold for paw withdrawal.
The paw withdrawal threshold in terms of %CBK was measured using a Randall-Selitto analgesiometer. The treatment with ALA, FA, and their combination significantly elevated the paw withdrawal threshold compared to non-treated diabetic rats [37]. In the present study, the mechanical hyperalgesia was evaluated using the Pinprick test, which showed a significant increase in the paw withdrawal threshold of the combination group compared to individual drugs in both treatment weeks. As for acetone-induced cold allodynia, the ALA and FA combination significantly elevated the paw withdrawal latency compared to the non-treated diabetic group. Additionally, the treatment with antioxidants combination normalizes the threshold in a better way compared to single antioxidant treatments. Change in nociception was well reported in earlier studies of diabetic neuropathic pain. The changes in the nervous system associated with neuropathic pain include a critical up-regulation of the calcium channel subunit CaVα2δ-1 [38]. PG effect mechanism includes the binding with α2δ subunit of voltage-gated calcium channel and leads to reduced release of neurotransmitters. These effects are linked to the attenuation of neuronal hyperexcitability and the development of analgesic effects [39].
STZ produces free radical species, thereby disturbing the endogenous balance between oxidants and antioxidants. In the present study, oxidative stress markers (LPO, GSH, and NO) were monitored to obtain a better idea of oxidative status. An increase in free radicals may react with a polyunsaturated fatty acid in the cell membrane leading to LPO formation; thus, the uncontrolled lipid peroxidation leads to damage to islet cells, which might be responsible for hyperglycemia [40]. Other studies showed that there was a high level of LPO in DN conditions [41]. GSH is a critical intracellular antioxidant, and its function is to suppress lipid peroxidation [44]. The decrease in GSH level and its metabolism impairments in the diabetic condition is due to competition between aldose reductase and glutathione reductase for NADPH and the increased oxidative stress [43][44]. The current study showed that ALA might have increased intracellular GSH levels. On the other hand, FA is a hydroxyl radical scavenger [45], which might have assisted under oxidative conditions. Increased levels of NO might be due to the stimulation of iNOS. Interaction of O2– with NO is rapid and leads to the formation of potent oxidant radical, i.e., peroxynitrite (ONOOˑ), which stimulates arachidonic acid metabolism, lipid peroxidation, and prostanoid production, leading to nonspecific tissue damage [46-48]. The NO level in the antioxidant combination group was significantly decreased as compared to DN. This observation might be the result of FA neutralizing NO radical and the reversing effect of ALA on NO- cGMP system as noted in previous studies; hence the decrease in the NO levels [46][49][50].
Membrane-bound enzymes (ATPase) are essential players in maintaining membrane integrity and thereby ionic balance. Na+/K+-ATPase is critical for the membrane potential and many types of transport. In the present study, membrane-bound ATPases (Na+/K+-ATPase, Ca2+-ATPase, and Mg2+-ATPase) were estimated in rat’s sciatic nerve homogenate and were found to be significantly altered in STZ-treated rats. The alteration in membrane-bound ATPases activities in the study might be due to the depletion of the intracellular pool of myo-inositol, increased flux through the aldose reductase pathway, and alteration in PKC [51]. Treatment with ALA or FA alone and in combination increased the level of ATPases compared to non-treated DN rats. The results were in line with previous reports [52]. ALA effect on Na+/K+-ATPase may be due to amelioration of nerve myo-inositol and taurine depletion, which could reflect on the restoration of Na+/K+-ATPase by Na+– dependent uptake [53-55].
It was found that ALA increased GLUT4 translocation to the cell membrane and increased glucose uptake in cultural adipose and muscle cells. It also has the capacity to scavenge ROS directly and regenerate endogenous antioxidants [19]. The mechanism of FA action as an antioxidant was due to its resonance stabilization. It neutralizes the free radicals produced by STZ in the pancreas, and it also increases insulin secretion, which helps lower blood glucose levels. Therefore, due to its antioxidant properties, FA has a neuroprotective effect. Therefore, scavenging free radicals reduced the above effects in sciatic pain models.
Conclusion
In the present study, the concomitant administration of ALA and FA in diabetic-induced neuropathic rats showed better effects compared to single ALA or FA treatments. These protective effects might be due to the potent antioxidant activities of the combined antioxidants, with each one of them affecting an independent pathway.
References
| 1 | Bhadada SK, Sahay RK, Jyotsna VP, Agrawal JK. Diabetic Neuropathy: Current Concepts. J Ind Clin Medi. 2001; 2(4):305-18. |
| 2 | Upaganlawar A. Supplementation of corosolic acid prevents the development of neuropathic pain in streptozotocin induced diabetic rats. J Pharmacol Clinic Sci.2016;1(1):11-8. |
| 3 | Horowitz SH. Textbook of diabetic neuropathy, edited by FA Gries, NE Cameron, PA Low, and D. Ziegler, Stuttgart, Thieme, 2003. Muscle & Nerve: Official Journal of the American Association of Electro diagnostic Medicine. 2004;30(2):247-. DOI |
| 4 | Taliyan R, Sharma PL. Diabetic Neuropathic Pain: An Update and Novel Pharmacological Strategies for Relief of Pain. J. Med. Sci. 2010;10(4):93-109. DOI |
| 5 | Kolgazi M, Jahovic N, Yüksel M, Ercan F, Alican I. α‐Lipoic acid modulates gut inflammation induced by trinitrobenzene sulfonic acid in rats. J. Gastroenterol. Hepatol. 2007;22(11):1859-65. DOI |
| 6 | Biewenga GP, Haene GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997;29:315-31. DOI |
| 7 | Bustamante J, Lodge JK, Marcocci L, Tritschler HJ, Packer L, Rihn BH. α-Lipoic acid in liver metabolism and disease. Free Rad. Biol. Med. 1998;24(6):1023-39. DOI |
| 8 | Packer L. α-Lipoic acid: a metabolic antioxidant which regulates NF-κB signal transduction and protects against oxidative injury. Drug Metab. Rev. 1998;30(2):245-75. DOI |
| 9 | Rochette L, Ghibu S, Richard C, Zeller M, Cottin Y, VergelyC.Direct and Indirect Antioxidant Properties of α-Lipoic Acid and Therapeutic Potential. Mol. Nutr. Food Res. 2013;57(1):114-25. DOI |
| 10 | Gajdosik A, Gajdosikova A, Stefek M, Navarova J, Hozova R. Streptozotocin-induced experimental diabetes in male Wistar rats. Gen. Physiol. Biophys. 1999;18:54-62. |
| 11 | Cory T. Pregabalin: latest safety evidence and clinical implications for the management of neuropathic pain. Ther Adv Drug Saf. 2014;5(1):38-56. DOI |
| 12 | Attal N, Cruccu G, Baron RA, Haanpää M, Hansson P, Jensen TS, Nurmikko T: EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113-e88. DOI |
| 13 | Rahaman H, Upaganlawar A, Upasani C. Protective effects of Ferulic acid alone and in combination with ascorbic acid on Aniline induced spleen toxicity. Anna of Pharmacol Pharmaceut. 2017. 2(1):1012-6. |
| 14 | Khairnar U, Upaganlawar A, Upasani C. Ameliorative effect of chronic supplementation of protocatechuic acid alone and in combination with ascorbic acid in aniline hydrochloride induced spleen toxicity in rats. Scientifica. 2016;2016. DOI |
| 15 | Upaganlawar A, Balaraman R. Cardioprotective effects of co-administration of Pomegranate extract and Vitamine E on electrocardiographic, biochemical and apoptotic changes in isoproterenol induced myocardial infarction in rats. Pharmacol; 2015;6(5):178-85. DOI |
| 16 | Upaganlawar A, Balaraman R. Combined effects of Vitamin E and lycopene on lipid profile and infarction size in isoproterenol induced cardiotoxicity in rats. Pharmacol. 2012; 3(7);215-20. |
| 17 | Raposo D, Morgado C, Pereira-Terra P, Tavaresa I. Nociceptive spinal cord neurons of laminae I–III exhibit oxidative stress damage during diabetic neuropathy which is prevented by early antioxidant treatment with epigallocatechin-gallate (EGCG). Brain Res. Bull. 2015;110: 68–75. DOI |
| 18 | Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53(1):55-63. DOI |
| 19 | Blackburn-Munro G, Ibsen N, Erichsen HK. A comparison of the anti-nociceptive effects of voltage-activated Na+ channel blockers in the formalin test. Eur. J. Pharmacol. 2002;445(3):231-8. DOI |
| 20 | Malcangio M, Tomlinson DR. A pharmacologic analysis of mechanical hyperalgesia in streptozotocin/diabetic rats. Pain. 1998;76(1-2):151-7. DOI |
| 21 | Levy D, Zochodne DW. Increased mRNA expression of the B1 and B2 bradykinin receptors and antinociceptive effects of their antagonists in an animal model of neuropathic pain. Pain. 2000;86(3):265-71. DOI |
| 22 | Yoon C, Wook YY, Sik NH, Ho KS, Mo CJ. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59(3):369-76. DOI |
| 23 | Kim KH, Tsao R, Yang R, Cui SW. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006;95(3):466-73. DOI |
| 24 | Saija A, Tomaino A, Trombetta D, De Pasquale A, Uccella N, Barbuzzi T, Paolino D, Bonina F. In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int. J. Pharm. 2000;199(1):39-47. DOI |
| 25 | Boyce-Rustay JM, Honore P, Jarvis MF. Animal models of acute and chronic inflammatory and nociceptive pain. InAnalgesia. Humana Press, Totowa, NJ. 2010;617:41-55. DOI |
| 26 | Yalcin IA, Charlet M, Freund-Mercier M, BarrotP.Differentiating Thermal Allodynia and Hyperalgesia Using Dynamic Hot and Cold Plate in Rodents. J. Pain .2009;10(7):767-73. DOI |
| 27 | Ohinishi TT, Suzuki Y, Suzuki K, Ozawa A. Comparative study of plasma membrane Mg2+ ATPase activities in normal, regenerating and malignant cells. Biochim Biophys Acta Biomembr. 1982;684(1):67-74. DOI |
| 28 | Slater TF, Sawyer BC. The stimulatory effect of carbon tetrachloride and other halogenalkane or peroxidative reaction in the rat liver functions in vitro. Biochem J. 1991; 123: 805-15. DOI |
| 29 | Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta Gen Subj. 1979;582(1):67-78. DOI |
| 30 | MISRA H. FridovichI. The role of superoxide anion in the autooxidation of epinephrine and a simple assay of SOD. J Bio Chem. 1972;247:3170-5. |
| 31 | Aebi H. Catalase in vitro. InMethods in enzymology. Academic Press. 1984;105:121-6 DOI |
| 32 | Hjertén S, Pan H. Purification and characterization of two forms of a low-affinity Ca2+ -ATPase from erythrocyte membranes. Biochim Biophys Acta Biomembr. 1983;728(2):281-8. DOI |
| 33 | Adisakwattana S, Moonsan P, Yibchok-Anun S. Insulin-releasing properties of a series of cinnamic acid derivatives in vitro and in vivo. J. Agric. Food Chem. 2008;56(17):7838-44. DOI |
| 34 | Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J. biol. Chem. 1925;66(2):375-400. |
| 35 | Busby R, Schelvis WJPM, Yu DS, Babcock GT, Marlett MA. Lipoic acid biosynthesis: LipA is an iron-sulphur protein. J Am Chem Soc. 1999;121(19):4706–7. |
| 36 | Singh U, Jialal I. Retracted: alpha-lipoic acid supplementation and diabetes. Nutr. Rev. 2008;66(11):646-57. DOI |
| 37 | Şengül M, Yildiz H, Kavaz A. The effect of cooking on total polyphenolic content and antioxidant activity of selected vegetables. Int. J. Food Prop. 2014;17(3):481-90. DOI |
| 38 | Verma V, Singh N, Singh Jaggi A. Pregabalin in neuropathic pain: evidences and possible mechanisms. Curr. Neuropharmacol. 2014 Jan 1;12(1):44-56. DOI |
| 39 | Hundehege P, Fernandez-Orth J, Römer P, Ruck T, Müntefering T, Eichler S, Cerina M, Epping L, Albrecht S, Menke AF, Birkner K. Targeting voltage-dependent calcium channels with pregabalin exerts a direct neuroprotective effect in an animal model of multiple sclerosis. Neurosignals. 2018;26(1):77-93. DOI |
| 40 | Pournaghi P, Sadrkhanlau R, Foroughi A. An investigation on body weight, blood glucose level and pituitary-gonadal axis hormones in diabetic and metformin-treated diabetic female rats. Vet Res Forum.2012;3(2):79-84. |
| 41 | Kumar N, Pruthi V. Potential applications of ferulic acid from natural sources. Biotechnol.2014;4: 86-93. DOI |
| 42 | Petya K. Improvement of insulin sensitivity in patients with type 2 diabetes mellitus after oral administration of alpha-lipoic acid. Hormones (Athens). 2014; 5(4):251-8. DOI |
| 43 | Choi R, Kim BH, Naowaboot J, Lee MY, Hyun MR, Cho EJ. Effects of ferulic acid on diabetic nephropathy in a rat model of type 2 diabetes. Exp. Mol. Med.2011;43(12):676-83. DOI |
| 44 | Howarth FC, Jacobson M, Shafiullah M, Adeghate E. Long‐term effects of streptozotocin‐induced diabetes on the electrocardiogram, physical activity and body temperature in rats. Exp. Physiol. 2005;90(6):827-35. DOI |
| 45 | Eze ED, Atsukwei D, Adams MD, Tende JA, Malgwi IS. Effects of alpha lipoic acid on blood glucose, body weight and haematological profile of streptozotocin-induced hyperglycaemia in wistar rats. Eur. J. Med. Res. 2015;3(2):25-33. |
| 46 | Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Therapeutic role of curcumin in prevention of biochemical and behavioral aberration induced by alcoholic neuropathy in laboratory animals. Neurosci. Lett. 2012;511(1):18-22. DOI |
| 47 | Bhokare KH, Upaganlawar AB. Neuroprotective effects of Lagerstroemia speciosa L. extract (Banaba Leaf Extract) in streptozotocin Induced painful diabetic neuropathy in laboratory rats. Pharmacologia. 2016;7:9-15. DOI |
| 48 | Jorige A, Annapurna A. Neuroprotective and antioxidant role of pregabalin in streptozotocin induced neurotoxicity. Int. J. Pharm. Sci. Res.. 2016;7(11):4494. |
| 49 | Finnerup NB, Jensen TS . Clinical use of pregabalin in the management of central neuropathic pain. Neuropsychiatr. Dis. Treat.2007;3(6):885–91. |
| 50 | Rajasekaran S, Sivagnanam K, Subramanian S. Antioxidant effect of Aloe vera gel extract in streptozotocin-induced diabetes in rats. Pharmacol Rep. 2005;57(1):90-6. |
| 51 | Ueno Y, Kizaki M, Nakagiri R, Kamiya T, Sumi H, Osawa T. Dietary glutathione protects rats from diabetic nephropathy and neuropathy. The Journal of nutrition. 2002;132(5):897-900. DOI |
| 52 | Kalkan IH, Suher M. The relationship between the level of glutathione, impairment of glucose metabolism and complications of diabetes mellitus. Pak J Med Sci. 2013;29(4):938. DOI |
| 53 | Bravenboer B, Kappelle AC, Hamers FP, Van Buren T, Erkelens DW, Gispen WH. Potential use of glutathione for the prevention and treatment of diabetic neuropathy in the streptozotocin-induced diabetic rat. Diabetologia. 1992;35(9):813-7. DOI |
| 54 | Budin SB, Othman F, Louis SR, Bakar MA, Radzi M, Osman K, Das S, Mohamed J. Effect of alpha lipoic acid on oxidative stress and vascular wall of diabetic rats. Rom J Morphol Embryol. 2009;50(1):23-30. |
| 55 | Ramar M, Manikandan B, Raman T, Priyadarsini A, Palanisamy S, Velayudam M, Munusamy A, Prabhu NM, Vaseeharan B. Protective effect of ferulic acid and resveratrol against alloxan-induced diabetes in mice. Eur. J. Pharmacol. 2012;690(1-3):226-35. DOI |
Cite this article:
Gupta, S., Sherikar, A., Upaganlawar, A., Upasani, C. Neuroprotective effects of α-Lipoic acid alone and in combination with ferulic acid in diabetic neuropathy induced rats. DYSONA – Life Science, 2020;1(3): 102-112. doi: 10.30493/dls.2020.243982
