Imen Mougou 1*
1, High Institute of Agronomy of Chott Mariem, 4042 Sousse, University of Sousse, Tunisia
E-mail:
imenmougou@gmail.com
Received: 10/01/2022
Acceptance: 05/02/2022
Available Online: 10/02/2022
Published: 01/04/2022

Manuscript link
http://dx.doi.org/10.30493/DLS.2022.323591
Abstract
Citriculture is considered one of the most important strategic agricultural sectors globally. Among citrus bacteriosis, blast and black pit caused by Pseudomonas syringae pv. syringae is ranked among top citrus bacterial diseases in terms of yield losses. Blast and black pit are widespread in several citrus-growing countries. The infection occurs when the bacterium invades injury sites. This bacterial organism produces symptoms on foliage and fruit. The blast symptoms appear on leaves and twigs, while those of black pit appear on the fruit. The bacterium prefers prolonged cool wet conditions. Currently, prophylactic measures, bactericide applications, the use of tolerant cultivars, and biological control are recommended for the management of citrus bacteriosis. This paper deals with citrus blast and black pit: symptoms, causative agent, epidemiology, disease cycle, and disease management.
Keywords: Pseudomonas syringae pv. syringae, Bacterial disease management, Citriculture
Introduction
Citrus is considered one of the major fruit crops worldwide [1]. This crop is subject to attacks by several phytopathogenic microorganisms such as bacteria, fungi, and viruses [2]. Although bacterial diseases are less frequent, these diseases may cause significant losses upon occurrence [3]. The primary bacterial diseases of citrus are citrus canker [4], bacterial spot [5], citrus variegated chlorosis [6], citrus greening [7],and blast/black pit disease [8-10].
The responsible agent of citrus blast and black pit is Pseudomonas syringae pv. syringae (Pss) bacterium [8-10]. The phytopathogenic bacteria Pseudomonas syringae (Ps) is a polyphagous bacteria associated with more than 180 species, including perennial and annual crops. The bacterial organisms were found as an epiphyte on the phyllosphere of several geographic locations [8-10].
During wet weather, citrus blast usually begins at a fresh wound or injury to the petiole wing and involves the petiole, base of the leaf blade, and a portion of the twig surrounding the base of the petiole. It may, at times, start in injuries at other points of the leaf or twig. The same bacterium that initiates blast is also responsible for the black pit on fruits. Therefore, Pss is considered an opportunistic pathogen since it enters plant tissue through wounds [11].
The black pit is most noticeable on lemon fruit but may also be found on oranges or other commercial cultivars [8-10]. Citrus blast and black pit cause severe damage in the orchard and consequently significant yield losses [9][10][12].
In this paper, the current knowledge of citrus blast and black pit diseases symptoms, epidemiology, and the impacts of biotic and abiotic factors on the disease development were summarized, and the possible management approaches were highlighted.
Citrus blast and black pit symptoms
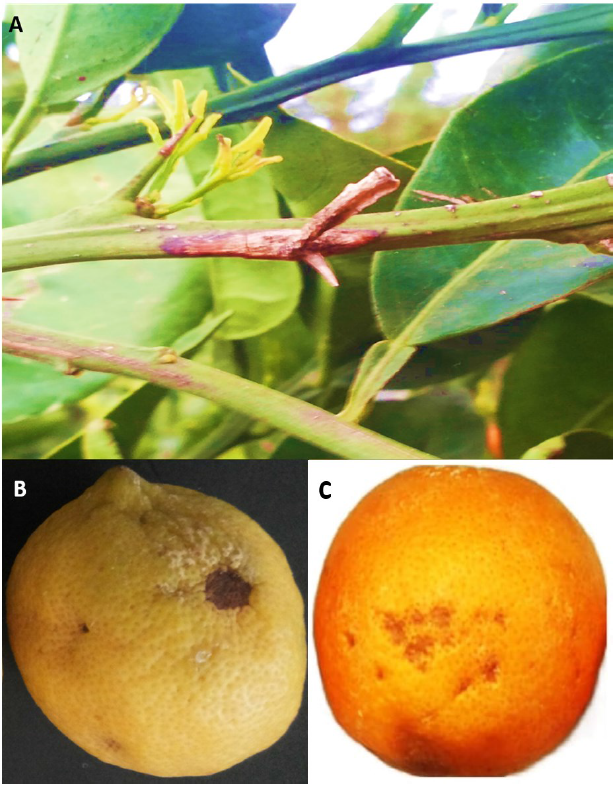
The lesions are first developed on the leaf petiole or wing of citrus as small water-soaked or dark spots. These lesions rapidly expand in the direction of the leaf midvein and the twig and axil tissues. The leaves wither, curl, dry, and become brown still attached or most often drop without petioles (Fig. 1 A). In reality, severe attacks result in entire twigs death. The withered dry leaves and dead twigs scattered throughout a tree canopy create the blasted appearance. Blast symptoms may be confused with frost damage [13].
Black pit appears on the fruit, especially lemons (Citrus sinensis L.) fruit which is the most susceptible to infection [9]. The bacterial organism can attack fruits suffering from physical injuries [14] due to hail or fraction with thorns and branches. The first symptom is a light brown spot on the fruit surface. These spots later become dark brown and eventually turn black and markedly sunken. Black pits may differ in size but are usually less than 1 cm in diameter. The pits may enlarge during postharvest storage and result in the loss of fruit (Fig. 1 B and C).
Causal agent of blast and black pit
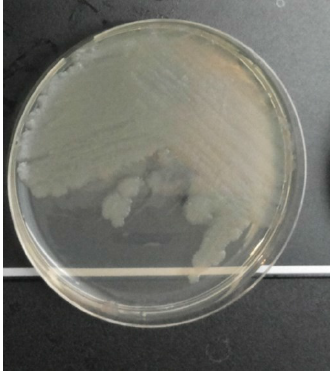
Pss bacterium (Fig. 2) is the causative agent of citrus blast and black pit [8-10]. Pss is an aerobic, Gram-negative, chemoorganotrophic bacterium and a member of the Gammaproteobacteria [15]. Under ultraviolet light, the bacterium produces fluorescent pigments, which is the pyoverdine characteristic of fluorescent Pseudomonads [16][17].
Epidemiology
The Pss bacterium is a normal inhabitant of citrus leaves and also many plant species [8]. The population levels of Pss strains which are associated with blast and black pit increase on the surface of citrus leaves and twigs during prolonged periods of fog or rain and low temperature. Blast and black pit occur mainly in regions where wet, cool, windy conditions often prevail during winter and spring [9]. Ps bacterium infects susceptible citrus tissues such as shoots and twigs, young succulent petioles, or fruit through wounds caused by hail, wind, or fractions [13][18]. Pss is a hemibiotrophic pathogen with biotrophic and necrotrophic characteristics since the bacterium obtains nutrients from living dead or dying host cells, respectively [19].
Weeds and plant debris were reported as possible inoculum sources [8]. Moreover, there is no evidence that blast and black pit result from Pss transmission via seeds, insects, contaminated equipment, or tools [13].
Disease cycle
The life cycle of Pseudomonas strains includes an epiphytic phase and a pathogenic phase. The epiphytic survival of the bacterium can be expressed by a significant epiphytic multiplication or the capacity to colonize plant phyllosphere. The epiphytic populations of Ps represent an inoculum source and are essential for disease infection [20]. Ps penetrate plant tissues through the injuries. Hail is considered a unique form of moisture that may contribute to the severity of the disease [21]. Citrus blast and black pit disease rarely progress when the temperature is below 21 °C [13]. However, citrus blast and black pit disease can quickly emerge under favorable conditions and may cause significant economic losses [22]. Reports from Turkey showed that the disease at a certain point affected 50 hectares of citrus and the disease incidence was about 100% [23].
Disease management
The protection of citrus orchards and nurseries against bacterial diseases is one of the most critical problems in fruit production. The most effective management of citrus bacteriosis is the prevention of infection. According to [13], the inoculum sources of Pss can be reduced by pruning dead or diseased twigs and shoots during the spring and after the rainy season. This procedure can reduce the incidence and the severity of citrus blast and black pit disease by minimizing infection and the spread of the pathogen. When possible, it is advisable to provide some means of protection against winds, such as installing windbreaks to reduce wind injuries, which is a major infection inducer. It is also necessary to monitor the nutritional status of the trees accompanied by a proper fertilization schedule and regular pruning to prevent excessive susceptible new growth in the fall [13].
The selection of resistant or tolerant cultivars is also an effective method of disease control [9]. Although no citrus cultivar is fully resistant to blast and black pit, few cultivars are less susceptible than others. [9] reported that lemon cultivar ‘Eureka’ was found least susceptible to citrus blast disease while clementine the cultivar ‘MA3’ and ‘Cassar’ tend to be less susceptible to black pit. Thus, these cultivars are recommended to replant affected citrus orchards, especially in cold and wet environments which promote citrus bacteriosis development. For new plantings, it is advisable to use bushy cultivars with relatively few thorns and, therefore, a minimum liability to injury during severe wind storms [9][13].
Chemical control of Ps is represented by a preventative application of copper compounds [24]. The timely copper spray application of protective copper-containing sprays such as Bordeaux mixture or fixed copper compounds is recommended in disease favorable conditions. Such spray in the fall and early winter before the first rains help to protect leaves and shoots from Pss infections in late winter and spring. An additional protective copper spray may be needed in mid to late winter if heavy or extended periods of rain occur [13].
In the susceptible cultivar or exposed areas, a high-wetting Bordeaux mixture is recommended for young plantings during October-November [25]. Special care in spraying should be taken to cover all the new growth of the previous year. In reality, copper treatments are very effective in controlling bacterial diseases [26-30], especially when mixed with other products, which increases copper solubility in the suspension [31] and therefore increases copper efficacity.
Although copper products are highly efficient in controlling bacterial pathogens, their application could lead to serious environmental and health problems in addition to the emergence of copper-resistant bacterial strains with continuous use. For these reasons, the biological control is highly recommended [10] [32-34]. Biological control is defined as the use of living organisms and their products to limit or suppress the activities and populations of pathogens [35]. Plant extract and some biological agents such as Bacillus spp. could play a prominent role in suppressing or controlling citrus bacteriosis [10] [36]. [10] reported that garlic extract and Bacillus spp. showed strong antimicrobial activity against the bacterial agent of citrus blast and black pit in vitro and under greenhouse conditions. Furthermore, a preliminary study [37] reported the use of phage treatment to control bacterial blast in citrus species. Therefore, biological control methods have high potential in controlling the pathogen, which still needs further research to exploit its economic feasibility.
Conclusions
Pseudomonas syringae pv. syringae is a typical inhabitant of citrus leaves and the causal agent of citrus blast and black pit. This bacterium affects both foliage and fruit. Disease symptoms occur under favorable conditions such as wet and cool windy weather, resulting in severe damage and yield losses in citrus groves. Weeds and plant debris may be a source of bacterium inoculum. The application of copper compounds, pruning, balanced nutritional program, the protection of trees against winds, and the use of less susceptible cultivars are recommended to control or prevent citrus blast and black pit disease. Furthermore, the exploitation of biological control treatments, such as plant extracts and antagonistic bacteria, is recommended to substitute the currently used chemical methods.
References
| 1 | Rehman A, Deyuan, Z, Hussain I, Iqbal M.S, Yang Y, Jingdong L. Prediction of Major Agricultural Fruits Production in Pakistan by Using an Econometric Analysis and Machine Learning Technique. Int. J. Fruit Sci. 2018;18:445–61. DOI |
| 2 | Dalio RJD, Magalhães DMM, Rodrigues CM, Arena GD, Oliveira TS, Souza-Neto RR, Picchi SC, Martins PMM, JC Paulo Santos, Maximo JH, Pacheco IS, De Souza AA, Machado MA. PAMPs, PRRs, effectors and R-genes associated with citrus–pathogen interactions. Ann. Bot. 2017;119(5):749–74. DOI |
| 3 | Sundin GW, Castiblanco L F, Yuan X, Zeng Q, Yang CH. Bacterial disease management: challenges, experience, innovation and future prospects. Mol. Plant Pathol. 2016.17: 1506–18. DOI |
| 4 | Golmohammadi M, Cubero J, Peñalver J, Quesada JM, López MM, Llop P. Diagnosis of Xanthomonas axonopodis pv. citri, causal agent of citrus canker, in commercial fruits by isolation and PCR-based methods. J Appl Microbiol. 2007;103:2309–15. DOI |
| 5 | Vauterin L, Hoste B, Kersters K, Swings J. Reclassification of Xanthomonas. Int J. Syst. Evol. Microbiol. 1995;45:472-89. DOI |
| 6 | Aguilar E, Villalobos W, Moreira L, Rodríguez CM, Kitajima EW, Rivera C. First Report of Xylella fastidiosa Infecting Citrus in Costa Rica. Plant dis. 2005;89:687. DOI |
| 7 | Bové JM. Huanglongbing: A destructive, newly emerging, century-old disease of citrus. J. Plant Pathol. 2006;88:7-37. DOI |
| 8 | Mougou I, Boughalleb-M’hamdi N. Detection, Survival, and Source of Inoculum of Pseudomonas syringe pv. syringae from Weeds and Plant Debris in Relation to Epidemiology of Bacterial Citrus Blast and Black Pit in Tunisia. Br Microbiol Res J. 2016; 16:1-10. DOI |
| 9 | Mougou I, Boughalleb-M’hamdi N. Differential susceptibility of citrus cultivars toward blast and black pit in Tunisia caused by Pseudomonas syringae pv. syringae. Eur J. Biotechnol. Biosci. 2016;4:17-24. |
| 10 | Mougou I, Boughalleb-M’hamdi N. Biocontrol of Pseudomonas syringae pv. syringae affecting citrus orchards in Tunisia by using indigenous Bacillus spp. and garlic extract. Egypt J. Biol Pest Control. 2018;28:60. DOI |
| 11 | Huang JS 1986. Ultrastructure of bacterial penetration in plants. Annu Rev Phytopathol.1986;24:141-57. DOI |
| 12 | Snowdon AL. A colour atlas of post-harvest diseases and disorders of fruit and vegetables. In: Snowdon AL (ed) General introduction and fruits. Wolfe Publishing, London .1990; 302 |
| 13 | Ferguson L, Grafton-Cardwell EE. “Citrus production manual”. UCANR Publications, Oakland, University of California. 2014;433. |
| 14 | Canfield ML, Baca S, and Moore LW. Isolation of Pseudomonas syringae from 40 cultivars of diseased woody plants with tip dieback in Pacific Northwest nurseries. Plant Dis. 1986;70:647-50. |
| 15 | Palleroni NJ. Family I. Pseudomonadaceae. In Hold JD (Ed.), Bergey’s Manual of Determinative Bacteriology. New York: 6th ed. Springer. 1994;110-199. |
| 16 | Lelliott RA, Billing E, and Hayward AC. A determinative scheme for the fluorescent plant pathogenic pseudomonads. J. Appl. Bacteriol. 1966;29:470-89. |
| 17 | Braun-Kiewnick A, Sands DC. 2001. Pseudomonas. In: Schaad ND, ed. Laboratory Guide for Identification of Plant Pathogenic Bacteria. USA: 3rd edn. St Paul, MN, APS Press. 2001;84-120. |
| 18 | Ivanović Z, Perović T, Popović T, Blagojević J, Trkulja N, Hrnčić S. Characterization of Pseudomonas syringae pv. syringae, Causal Agent of Citrus Blast of Mandarin in Montenegro. Plant Pathol. J. 2017.33(1):2133. DOI |
| 19 | Collmer A, Schneider DJ, and Lindeberg M. Lifestyles of the effector rich: genome-enabled characterization of bacterial plant pathogens. Plant Physiol. 2009;150:1623-30. DOI |
| 20 | Gorlenko MV. Bacterial diseases of plants. Army Biological Labs Fredrick MD; 1968. |
| 21 | Beiki F, Busquets A, Gomila M, Rahimian H, Lalucat J, Garcia-Valdes E. New Pseudomonas spp. are pathogenic to Citrus. PLoS ONE. 2016;11:1–16. DOI |
| 22 | Gaignard JI, Luisetti J. Pseudomonas syringae, bactérie épiphyte, glaçogène et pathogène. Agronomie. 1993;13(5):333–70. DOI |
| 23 | Mirik M, Baloglu S, Aysan Y, Cetinkaya-Yildiz R, Kusek M, Sahin F. First outbreak and occurrence of citrus blast disease, caused by Pseudomonas syringae pv. syringae, on orange and mandarin trees in Turkey. Plant Pathol. 2005;54:238. DOI |
| 24 | Montesinos E, Lopez MM. Métodos de control de las bacteriosis. In Llacer G, Lopez MM, Trapero T, Bello A (Eds.). Espana: Patologia Vegetal. Tomo 1. Societad Espanola de Fitopatologia. 1996;653-78. |
| 25 | Camille J, Franck C, Marion H. 2013. Les clémentiniers et autres petits agrumes. France: Ed. Quae. 2013;370. |
| 26 | Marco GM, Stall RE. Control of bacterial spot of pepper initiated by strains of Xanthomonas campestris pv. vesicatoria that differ in sensibility to copper. Plant Dis. 1983;67:779-81. DOI |
| 27 | Lavermicocca P, Lonigro SL, Valerio F, Evidente A, Visconti A. Reduction of Olive Knot Disease by a Bacteriocin from Pseudomonas syringae pv. ciccaronei. Appl. Environ. Microbiol. 2002;68(3):1403-07. DOI |
| 28 | Luisetti J, Gaignard JL, Vigouroux A, Saunier R, Lafuste JP, Charras J. Pêcher, le dépérissement bactérien. Arboric Fruit. 1992;447:19-28. |
| 29 | Benjama A. Les maladies parasitaires de l’olivier au Maroc. II année-N20. Edition Française. 1988;133. |
| 30 | Teviotdale B L, Krueger WH. Effects of Timing of Copper Sprays, Defoliation, Rainfall, and Inoculum Concentration on Incidence of Olive Knot Disease. Plant. Dis. 2004;88:131-5. DOI |
| 31 | Marco GM, Stall RE. Control of bacterial spot of pepper initiated by strains of Xanthomonas campestris pv. vesicatoria that differ in sensibility to copper. Plant Dis.1983;67:779-81. DOI |
| 32 | Fravel DR. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005;43:337-59. DOI |
| 33 | Stewart A, Hill R, Stark C. 2011. Desktop evaluation on commercially available microbial-based products for control or suppression of Pseudomonas syringae pv. actinidiae. Lincoln Universit: Bio-Protection Research Centre. 2011;33. |
| 34 | Suo Y, Leung DWM. Induction of resistance to Diplocarpon rosae and Agrobacterium tumifaciens by acibenzolar-S-methyl (BTH) in rose. Z. Pflanzenkrankh. Pflanzenschutz. 2001;108(4):382-91. |
| 35 | Dekker M, 2002. Biological Control of Crop Diseases. New York: CRC Press. 2002;468. |
| 36 | Islam MS, Sultana R, Hasan MA, Alam MS, Sikdar B, Kamaruzzaman M, Islam MA. Characterization and biocontrol measures of Pseudomonas syringae pv. syringae associated with citrus blast disease. Vegetos. 2020;33:555–69. DOI |
| 37 | Pinheiro LAM , C P, Pereira C, Frazão C, Balcão VM, Almeida A. Efficiency of Phage φ6 for Biocontrol of Pseudomonas syringae pv. syringae: An in Vitro Preliminary Study. Microorganisms. 2019;7(9):286. DOI |
Cite this article:
Mougou, I. Citrus blast and black pit disease: A review. DYSONA – Life Science, 2022;3(1): 1-6. doi: 10.30493/dls.2022.323591
