Emad E. Al-Drisi 1; Majid Ibrahim 1*; Abbas M. Jasim 1
1, College of Agriculture, University of Basrah, Basrah, Iraq
E-mail:
majid.abdulhameedl@uobasrah.edu.iq
Received: 26/09/2022
Acceptance: 06/11/2022
Available Online: 08/11/2022
Published: 01/01/2023

Manuscript link
http://dx.doi.org/10.30493/DAS.2022.363580
Abstract
This study intends to advance the understanding of papaya micro-propagation in order to minimize the commercial production costs of healthy papaya seedlings. For this purpose, in vitro growth behavior of different types of explants (shoot tips, nodule stem segments, internode segments, young leaves, petioles, and floral buds) from the hybrid papaya cultivar ‘Red Lady’ were studied and monitored after culturing on Murashige and Skoog medium containing 0.5 mg L-1 benzyl adenine and 0.1 mg L-1 Naphthaleneacetic acid. The results showed apparent differences between explant sources regarding the ability to induce, the speed of formation, and the weight of the resulting callus. The explants derived from the shoot tips produced a callus after eight days of culturing, while callus induction from young leaves required 21 days. After four weeks of culture, the highest fresh callus weight was obtained from the shoot tips and the stem nodules, which were 5.17 and 5.24 g, respectively. It is worth mentioning that the obtained callus texture was friable and grainy in all explants. The indirect shoots were induced from the shoot tips and stem nodules callus after four weeks of culturing at rates of 2.5 and 3.03 shoot per explant, respectively. No indirect shoots were obtained from the young leaves and petiole callus. The floral bud cultures resulted in young fruits without callus induction, followed by the regeneration of direct shoots after four weeks of culturing at the rate of 22.1 shoots per explant.
Keywords: Carica papaya, In vitro, Explant, Callus induction, Organogenesis
Introduction
Papaya (Carica papaya L.) is a tropical and subtropical fruit plant belonging to the family Caricaceae, which is native to southern Mexico and South America [1]. The global production of papaya fruit in 2020 reached 13.9 million tons. The major papaya-producing countries are India, the Dominican, Brazil, Mexico, Indonesia, and Nigeria [2]. Many studies have found that papaya seeds, leaves, and fruits can help prevent arthritis, lower cholesterol, slow aging, reduce the risk of cardiovascular diseases, assist weight loss, and aid digestion [3][4].
Papaya plants reproduce mainly by seeds. This traditional method leads to genetic variation and the production of seedlings that are not true-to-type and susceptible to viral infections. Several hybrid papaya cultivars were produced to obtain F1 plants with desirable fruit size, quality, and pest resistance [5]. Nevertheless, the F2 plants show genetic differences that lead to a loss of productivity and quality standards. Therefore, vegetative propagation of papaya using the tissue culture technique is necessary to preserve genetic assets and hybrid cultivars [6].
Many researchers have emphasized the significance of the source and differentiation stage of the explants employed in multiplication for successful establishment in vitro [7-10]. The immature or less differentiated explants are the easiest and fastest to initiate cell division [11]. This observation could be attributed to the high activity of these tissues and the high levels of endogenous hormones [12]. Many researchers have also indicated the possibility of optimizing the tissue culture workflows by selecting appropriate explants such as leaves, petioles, cotyledonary leaves, shoot tips, buds, embryos, and roots [8][13-15].
Many studies [6][16-22] investigated the shoot generation potentials of various explant types of papaya cultivars on culture media supplemented with various growth regulators. In this context, this research investigates in vitro growth behavior for different types of explants (shoot tips, nodule stem segments, internode segments, young leaves, petioles, and floral buds) of ‘Red Lady’ hybrid papaya cultivar.
Material and Methods
Tissue culture
The experiment was conducted in the tissue culture laboratory of Fadak Company for Animal and Plant Production in Al-Basrah governorate, Iraq. The explants were obtained from two-month seedlings of hybrid papaya cv. ‘Red Lady’ (Shoot tips, stem nodules, internode, young leaves, and petioles). Floral buds were also obtained from six-month seedlings.
Surface disinfection was accomplished by washing the explants with distilled water three times and immersing them in mercury chloride (HgCl2) 0.01% with three drops of Tween 20 for 10 min. After that, the explants were washed well with distilled water three times.
The culture medium was prepared with 4.43 gL-1 Murashige and Skoog (MS) salts [23] and vitamins (Caisson Labs Company, USA), 0.5 mg L-1 benzyl adenine (BA), 0.1 mg L-1 Naphthaleneacetic acid (NAA), 30 g L-1 Sucrose, and 8 g L-1 agar (HIMEDIA Company, India). The prepared MS medium was poured at 20 ml per culture jar and autoclaved at 1.05 kg cm-2 and 121 °C for 18 minutes. Afterward, the sterilized explants were cultured on the prepared MS medium and incubated in the chamber at 25±2 °C and 16/8 hour of light/darkness cycles provided by LED white fluorescent.
Growth behavior and characteristics
Culture growth behavior and characteristics were recorded as follows:
The period of callus emergence (days) was recorded when the first callus emergence was observed in any replicate of each treatment (explant type).
Fresh callus weight was measured after four weeks of culturing (g).
Callus texture and color were observed after four weeks of culturing.
Shoots Number (shoots per explant) and length (cm) were measured after four weeks of culturing.
Experimental design and statistical analysis
The experiment was conducted in a complete randomized design (CRD) with ten replications for each treatment (explant type). Data were analyzed using Genstat software. Means were compared using Fisher’s least significant difference test (LSD) at a probability level of 1%.
Results and Discussion
Callus induction
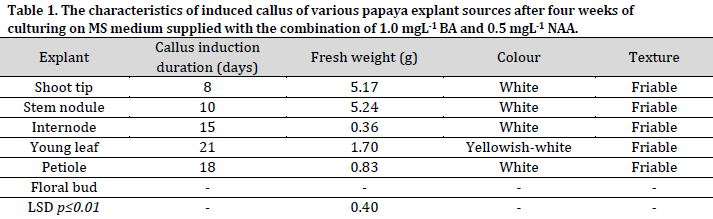
Callus was induced in all used explants except the floral buds that developed into small parthenocarpic fruits. This result agrees with the [24], who obtained parthenocarpic fruits from the floral buds. This observation could be attributed to the endogenous hormone content in floral buds and the exogenous plant growth regulators applied to the used MS medium, which led to ovaries enlargement [25][26].
Drastic differences in callus induction time were observed between the used explants. The shortest and longest callus induction periods were observed in shoot tips (8 days) and young leaves (21 days), respectively. The difference in the ability of callus induction and the duration of its formation in different explant types might be due to the different concentrations of plant hormones in these tissues since these hormones play a crucial role in cells differentiation and division that leads to the formation of new cells and induction of the callus tissue [8-10][22]. Furthermore, the used combination of auxin and cytokinin concentration may have promoted cell division and callus induction differently in the different tissues [6][27][28].
Significant differences were observed between the explants regarding the fresh weight of induced callus (Table 1). After four weeks of culturing, the highest fresh weight of callus was recorded in stem nodules (5.24g) and shoot tips (5.17g) explants, with no significant differences observed between the two sources. On the other hand, the internodes recorded the lowest fresh callus weight with 0.36g. These results were in agreement with the results previously reported in [29], which showed that the fresh weight of the stem nodules callus was higher compared to the callus induced by petiole culture. The texture of the induced callus from all explants in this study was white with a friable and granular texture, consistent with other reports [30].
Direct and indirect organogenesis
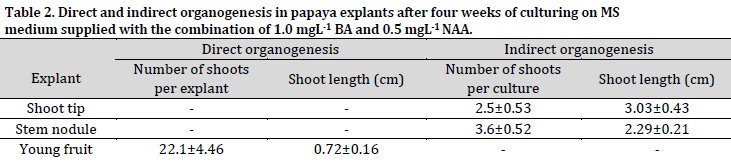
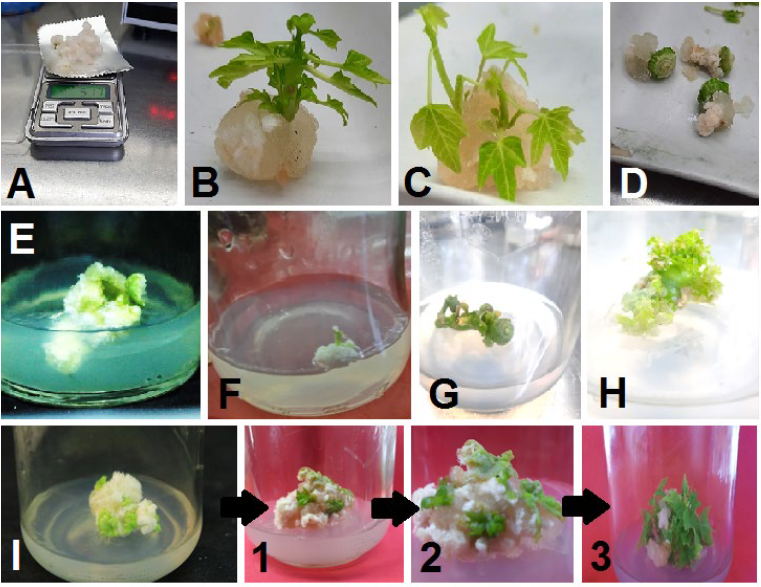
Shoot indirect organogenesis (from induced callus) was observed in shoot tips and stem nodules explants after four weeks of culturing (Fig. 1 B and C). On the other hand, no shoot formation was observed in internode, young leaves, and petiole explants at the same inspection date (Fig. 1 D, E, and F). The immature fruits were cultured on the same medium, leading to the regeneration of direct shoots after four weeks of culturing (Fig. 1 H). This phenomenon can be attributed to the young fruit and medium content of plant growth regulators, which led to the stimulation of cell division and development on the outer surfaces of fruits into direct shoots. The type of organogenesis in the immature papaya fruits contradicted the result of [27], who reported that the young strawberry fruits only induced a callus under in vitro conditions.
The average number of indirect shoots induced from the shoot tips and stem nodules callus was 2.5 and 3.6 shoots per culture, respectively, while the direct shoots resulted from the floral buds, which developed into young fruits, were 22.1 shoots per explant (Table 2).
On the other hand, the average lengths of the indirect shoots regenerated from the shoot tips and stem nodules callus were 3.03 cm and 2.29 cm, respectively, while the shoots generated from the young fruit explants via direct organogenesis were 0.72 cm on average. These findings show a negative relationship between the length and prevalence of the generated shoots from each explant.
Conclusion
All explants of the hybrid papaya ‘Red Lady’ led to callus induction, except for the floral buds that developed into the parthenocarpy fruits. The shortest period of callus induction was observed in shoot tip explants, where callus formation was observed after eight days of culturing. The highest fresh callus weight after four weeks of culture was obtained from the shoot tips and the stem nodules. Indirect shoot organogenesis was recorded from the callus of shoot tips and stem nodules explants after four weeks of culturing, while the immature young fruits regenerated direct shoots without callus induction.
Conflict of interest statement
The authors declared no conflict of interest.
Funding statement
The authors declared that no funding was received in relation to this manuscript.
Data availability statement
The authors declared that all related data are included in the article.
References
| 1 | Burns P, Saengmanee P, Doung-ngern U. Papaya: The Versatile Tropical Fruit. In: Tropical Plant Species. IntechOpen. 2022:1-14 DOI |
| 2 | FAO, FAOSTAT. Food and Agriculture Organization of the United Nations, Rome. 2020 Link |
| 3 | Koul B, Pudhuvai B, Sharma C, Kumar A, Sharma V, Yadav D, Jin JO. Carica papaya L.: A Tropical Fruit with Benefits beyond the Tropics. Diversity. 2022;14(8):683. DOI |
| 4 | Ibrahim M. Role of Endogenous and Exogenous Hormones in Bioactive Compounds Production in Medicinal Plants Via In Vitro Culture Technique. In: Plant Hormones: Recent Advances, New Perspectives and Applications. IntechOpen. 2022:131-47. DOI |
| 5 | Subramanyam MD, Iyer CP. Exploitation of heterosis in papaya. Indian J. Hortic. 1984;41(1,2):40-6. |
| 6 | Waidyaratne SM, Samanmalie LG. In vitro Propagation of Carica papaya L. Variety ‘Horana Papaya Hybrid 01’Using Shoot Tip. World. 2022;10(1):15-9. DOI |
| 7 | Suthamathi SJ, Haripriya K, Kamalakannan S. Micropropagation in papaya cv. CO-5. Indian J. Hortic. 2002;59(1):13-5. |
| 8 | Kumar N, Reddy MP. In vitro plant propagation: a review. J. for. environ. sci.. 2011;27(2):61-72. DOI |
| 9 | Ibrahim MA, Al-Taha H, Seheem AA. Effect of cytokinin type and concentration, and source of explant on shoot multiplication of pineapple plant (Ananas comosus‘ Queen’) in vitro. Acta Agric. Slov. 2013;101(1):15-20. DOI |
| 10 | Ibrahim MA, Draaj IA. The effect of explant source and cytokinin concentration on the direct bulb formation of tulip (Tulipa gesnerina L.) by plant tissue culture technique. Plant Cell Biotechnol. Mol. Biol. 2020;21(41,42):111-9. |
| 11 | Reuveni O, Shlesinger DR, Lavi U. In vitro clonal propagation of dioecious Carica papaya. Plant Cell, Tissue Organ Cult. 1990;20(1):41-6. DOI |
| 12 | Tyagi AP, Comai L, Byers B. Comparison of plant generation from root, shoot, and leaf explants in Pigeon pea (Cajanus cajan) cultivars. SABRAO J. Breed. Genet. 2001;33:59-72. |
| 13 | Kumar N, Vijay Anand KG, Reddy MP. Plant regeneration of non-toxic Jatropha curcas—impacts of plant growth regulators, source and type of explants. J. Plant Biochem. Biotechnol. 2011;20(1):125-33. DOI |
| 14 | Usman MU, Fatima B, Jaskani MJ, Iqbal MA. Development of a callogenic protocol in papaya (Carica papaya L.). 2. In vitro grown vegetative explants. Int. J. Agric. Biol. 2002;4:99-102. |
| 15 | Van Droogenbroeck B, Breyne P, Goetghebeur P, Romeijn-Peeters E, Kyndt T, Gheysen GA. AFLP analysis of genetic relationships among papaya and its wild relatives (Caricaceae) from Ecuador. Theor. Appl. Genet. 2002;105(2):289-97. DOI |
| 16 | Agnihotri S, Singh SK, Jain M, Sharma M, Sharma AK, Chaturvedi HC. In vitro cloning of female and male Carica papaya through tips of shoots and inflorescences. Indian J. Biotechnol. 2004;3:235-40. |
| 17 | Koehler AD, Carvalho CR, Abreu IS, Clarindo WR. Somatic embryogenesis from leaf explants of hermaphrodite Carica papaya: A new approach for clonal propagation. Afr. J. Biotechnol. 2013;12(18):2386-91. |
| 18 | Setargie A, Mekbib F, Abraha E. In vitro propagation of papaya (Carica papaya L.). World J. Agric. Sci. 2015;11(2):84-8. DOI |
| 19 | Efendi D, PutraB MR. Optimation of in vitro lateral shoots multiplication of papaya (Carica papaya L.)“Callina” with BAP and NAA. J. Trop. Crop Sci. 2017;4(3). DOI |
| 20 | Ryavalad S, Malabasari TA, Shantappa T, Uppar DS, Biradar BD, Mantur SM. Micropropagation Studies in Papaya (Carica papaya L.) cv.‘Surya’. Int J Curr Microbiol Appl Sci. 2019;8(4):2362-7. DOI |
| 21 | Cipriano JL, Cruz AC, Mancini KC, Schmildt ER, Lopes JC, Otoni WC, Alexandre RS. Somatic embryogenesis in Carica papaya as affected by auxins and explants, and morphoanatomical-related aspects. An. Acad. Bras. Cienc. 2018;90:385-400. DOI |
| 22 | Solórzano-Cascante P, Sánchez-Chiang N, Jiménez VM. Explant type, culture system, 6-benzyladenine, meta-topolin and encapsulation affect indirect somatic embryogenesis and regeneration in Carica papaya L. Front. Plant Sci. 2018;9:1769. DOI |
| 23 | Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15(3):473-97. DOI |
| 24 | Cohen JD. In vitro tomato fruit cultures demonstrate a role for indole-3-acetic acid in regulating fruit ripening. J. Am. Soc. Hortic. Sci. 1996;121(3):520-4. DOI |
| 25 | Su L, Rahat S, Ren N, Kojima M, Takebayashi Y, Sakakibara H, Wang M, Chen X, Qi X. Cytokinin and auxin modulate cucumber parthenocarpy fruit development. Sci. Hortic. 2021;282:110026. DOI |
| 26 | Sharif R, Su L, Chen X, Qi X. Hormonal interactions underlying parthenocarpic fruit formation in horticultural crops. Hort. Res. 2022;9. DOI |
| 27 | Ikeuchi M, Sugimoto K, Iwase A. Plant callus: mechanisms of induction and repression. Plant Cell. 2013;25(9):3159-73. DOI |
| 28 | Malik N, Sengar RS, Yadav MK, Singh SK, Singh G, Kumar M. Effect of Different Plant Growth Regulators on In-vitro Callus Induction in Carica papaya (cv. Pusa Nanha). Int. J. Curr. Microbiol. Appl. Sci. 2019;8(7):1217-25. DOI |
| 29 | Nasir MI. In vitro regeneration and acclimatization of Carica papaya L. (Doctoral dissertation), University of Pendidikan Sultan Idris, Malaysia. 2018:104. |
| 30 | Hong YC, Read PE, Harlander SK, Labuza TP. Development of a tissue culture system from immature strawberry fruits. J. Food Sci. 1989;54(2):388-92. DOI |
Cite this article:
Al-Drisi, E., Ibrahim, M., Jasim, A. Explant type influences callus induction and shoots organogenesis in papaya under in vitro conditions. DYSONA – Applied Science, 2022;4(1): 15-20. doi: 10.30493/das.2022.363580
