Oluwafemi A. Agbetuyi 1, 2*; Anthony H. Ekeocha 1; Ademiju A. Aganga 1
1, Department of Animal Production and Health, Faculty of Agriculture, Federal University Oye Ekiti, Ekiti State, Nigeria
2, Department of Agriculture and Food Security, Oye Local Government, Oye Ekiti, Ekiti State, Nigeria
E-mail:
femzyright@gmail.com
Received: 11/04/2024
Acceptance: 30/06/2024
Available Online: 30/06/2024
Published: 01/07/2024

Manuscript link
http://dx.doi.org/10.30493/DAS.2024.451989
Abstract
This study evaluated the physical and chemical properties and lipid profile of the Pectoralis major muscle in broiler chickens (Cobb 500) fed diets supplemented with Moringa oleifera leaf powder (MLP) and Allium sativum bulb powder (ABP). A total of 240 1-day-old broilers were assigned to five dietary treatments in a completely randomized design (CRD), with four replicates per treatment (12 birds/replicate). The dietary groups were: T1 (Control) without MLP and ABP, T2 with MLP replacing soybean meal (SBM) at 1%, T3 with MLP replacing SBM at 3%, T4 with MLP replacing SBM at 1% + 0.1% ABP, and T5 with MLP replacing SBM at 3% + 0.3% ABP. Diets were provided ad libitum from day 8 to day 56. The study consisted of an initial 28-day phase and a subsequent 28-day phase, after which the Pectoralis major was collected for analysis. On day 28, treatments T5 and T2 showed significantly higher moisture content (MC) and water-holding capacity (WHC) compared to the control, aligning with T4. By day 56, no significant differences in MC and WHC were observed across treatments. Total cholesterol and low-density lipoprotein (LDL) levels were significantly lower in T1, while greater high-density lipoprotein (HDL) levels were observed compared to other treatments except T5. Therefore, the combined garlic and moringa supplementation (T5) can positively enhance some of the cooking characteristics of broiler meat (such as moisture content) while maintaining a dietary healthier lipid profile.
Keywords: Moringa oleifera, Allium sativum, Feed additives, Lipid profile, Moisture content
Introduction
Phytogenic feed additives, encompassing plant parts, essential oils, and extracts, contain both volatile and non-volatile phyto-constituents that can be utilized in organic poultry nutrition to enhance the health status of birds and increase their productivity [1]. These additives are increasingly employed by farmers due to their ability to improve poultry performance and enrich products with natural antioxidants [1]. Moreover, their antimicrobial constituents are effective in combating various known infectious diseases in humans and animals [2]. Enhancing the quality of poultry meat can be significantly achieved through the manipulation of the dietary feed intake of the birds [3]. Notable plants and extracts that have attracted research attention in the poultry subsector include Moringa oleifera, Allium sativum [4-6], and shea butter [7].
Moringa oleifera application in livestock feeding is currently under investigation for its rapid growth, high nutritional value [8]. This plant contains over 90 components with antioxidant properties, including all eight essential amino acids [9]. Research indicates that incorporating M. oleifera leaf extract as a feed supplement enhances feed intake in poultry [10]. Supplementation in broiler diets has been associated with increased serum high-density lipoprotein (HDL) levels and reduced serum low-density lipoprotein (LDL) levels [5]. Moreover, increased supplementation of M. oleifera in broiler diets has been linked to a decrease in total cholesterol [6]. The leaves are rich in phytobiotics such as carotenoids, tocopherols, phenolics, and ascorbic acid, all of which are valuable dietary antioxidants [11]. Its high levels of protein, minerals, β-carotene, and antioxidants can be beneficial for human and animal nutrition [12]. Inclusion of Moringa oleifera leaf meal at 12 g/kg has been shown to increase pH, water-holding capacity, and muscle fiber diameter in the breast muscle of broilers [13]. Therefore, dietary supplementation with antioxidant-rich M. oleifera leaves is a promising strategy for enhancing meat quality in broiler chickens. Despite its significant economic potential, Moringa oleifera remains underexploited and is considered underutilized [14].
Allium sativum (garlic) is a globally recognized herb and spice known for its medicinal properties and has been extensively utilized in various applications, including animal production [15]. It contains several phytochemical substances such as flavonoids, saponins, tannins, alkaloids, steroids, hydrocyanides, and anthocyanins. According reports, the flavonoid, saponin, and tannin contents in Allium sativum range from 0.04% to 0.36%, 0.14% to 19.0%, and 0.06% to 6.10%, respectively [16]. A. sativum exhibits numerous beneficial effects, including antimicrobial, hypolipidemic, antihypertensive, anti-atherosclerotic, antidiabetic, antiviral, and antifungal properties [17][18]. Its bioactive compounds are diverse, with allicin being the primary component [19]. Allicin is capable of reducing low-density lipoprotein (LDL), triglyceride, and cholesterol levels in the serum and tissues of broiler chickens [20], leading to improved overall performance in birds. Research indicated that Allium sativum exerts hypocholesterolemic effects through the inhibition of key enzymes involved in cholesterol and lipid synthesis, such as 3-hydroxy-3-methylglutaryl-coenzyme A reductase, cholesterol-7α-hydroxylase, and those involved in fatty acid synthesis [21]. The hypocholesterolemic effects of Allium sativum, along with ginger, have been shown to reduce fat content in broiler meat, enhancing its safety for human consumption [22].
Incorporating natural plant extracts such as Moringa oleifera and Allium sativum into poultry diets has been reported to improve the oxidative stability, shelf-life, and color of meat [23], attributed to the presence of flavonoids and other phenolic compounds with antioxidative and hypocholesterolemic properties. Dietary antioxidants are believed to modify meat color, minimize rancidity, and reduce lipid peroxidation, thereby maintaining meat quality. The oxidative status of muscle tissue is directly related to its quality [24]. Consequently, this research aims to evaluate the physico-chemical characteristics and lipid profile of the Pectoralis major muscle in broiler chickens subjected to dietary feeding with Moringa oleifera and Allium sativum.
Materials and Methods
Plant material preparation
Moringa oleifera leaves were collected from the Moringa plantation located at the experimental site of the Crop Science Department, Teaching and Research Farm, Federal University, Oye Ekiti, Ikole Campus, Nigeria. Allium sativum bulbs were sourced from a local market (Shasha) in Ado Ekiti, Ekiti State, Nigeria. The collected Moringa leaves were allowed to air-dry for 7 days under shaded and well-ventilated conditions. Once dried, the leaves were ground using a mortar and pestle to produce Moringa leaf powder (MLP), which was subsequently stored in a plastic airtight container until needed for the experiment. Allium sativum bulbs were segmented into cloves, which were then cut into chips. These chips were sun-dried for 2 weeks until they became crispy. The dried chips were ground with a mortar and pestle to produce Allium sativum bulb powder (ABP), which was stored in an airtight container for use in the feeding trial.
Experimental site
The experiment was conducted at the Federal University, Oye Ekiti, Ekiti State, Nigeria at Animal Production and Health Research Unit, Ikole Ekiti (Located at latitude of 7.7982661°N and longitude of 5.514493°E). It has the annual average temperature of 24.2 °C.
Experimental management
Before the arrival of the day-old chicks, the experimental pens with a dimension of 1.83 × 1.22 m (length×breadth) were cleaned, fumigated and disinfected with potassium permanganate and germicide (Izal). Wood shavings were spread on the floor to a depth of 15.24 cm as bedding materials for the birds, and were changed every two weeks. A foot dip was provided at the entrance of the poultry house for disinfection of the foot-wears of those entering the pen. An anti-Stress pack (glucose) was administered through water on the day of arrival. Routine management practices were carried out in all the replicates. Proper hygiene and bio-security measures were ensured throughout the experimental period.
Feed preparation
Five experimental diets were formulated and made into homogenous diets manually with Moringa oleifera leaf powder and Allium sativum bulb powder incorporated to obtain the fine mash given to the birds. The feed additives (test ingredients) were first mixed thoroughly with other micro minerals for 1 minute, then one kilogram of grounded maize was added to the homogenized and upturned together for another 1 minute. These were later added to other ingredients that had been measured and were then mixed on the floor with a spade for another 15 minutes to obtain a 100 kg of the experimental diet. The conventional ingredients were purchased from Unique Feed mill, Ado Ekiti, Ekiti State, Nigeria.
Experimental design
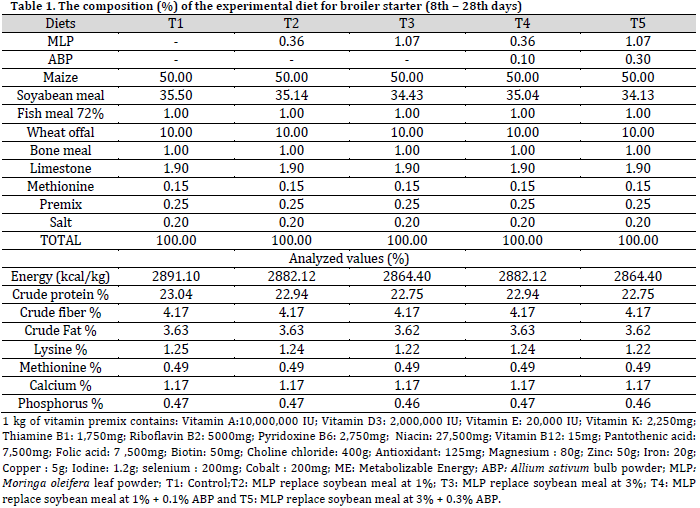
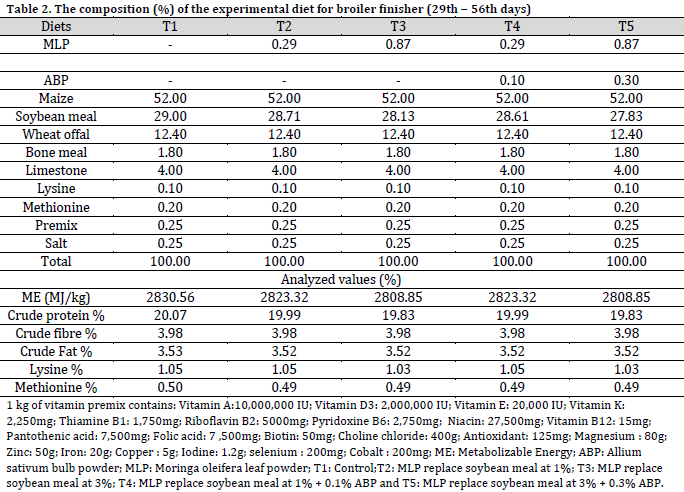
A total of two hundred and forty (n=240), 1-day-old unsexed (Cobb 500) broiler chickens were used for the experiment, which were randomly assigned to 5 dietary treatments with 4 replicates (12 birds/replicate). A basal diet was prepared to meet all the nutritional requirements of the broiler chickens. The birds were fed the basal diet ad libitum for days 1-7 of age. The basal diet was divided into 5 portions and the soybean meal proportion of each experimental diet was replaced with MLP and ABP, as follows: T1: Control, T2: MLP replace soybean meal at 1%; T3: MLP replace soybean meal at 3%, T4: MLP replace soybean meal at 1% + 0.1% ABP, and T5: MLP replace soybean meal at 3% + 0.3% ABP. The experimental diets and water were offered ad libitum from the 8th to 56th day of age. The design of the experiment was completely randomized design (CRD). The ingredients and chemical composition of both experimental starter and finisher diets were presented in Table 1 and 2.
Collection of data
Four birds weighing close to the average weight of each replicate were randomly selected (On the 28th day and 56th day) and slaughtered by cutting the jugular vein. The birds were tagged and fasted overnight before slaughtering. The slaughtered birds were scalded at 80 °C for 2 minutes and manually defeathered. The carcass was carefully eviscerated and split open to remove the gastrointestinal tracts. The breast muscle (Pectoralis major) samples were collected from each bird for further inspections.
Physico-chemical characteristics
The physico-chemical characteristics of the birds were performed inside the Laboratory of the Department of Animal Production and Health, Faculty of Agriculture, Federal University Oye Ekiti, Ikole Campus, Ekiti State, Nigeria.
Moisture content
The moisture content (MC) of the broiler meat was assessed through “Loss on drying method”. 50g of breast muscle (Pectoralis major) samples from each carcass were collected, placed in a carbolite laboratory oven (Model: LHT 5/30), and heated at 73.9 °C for 40 minutes. The dried meat samples were then weighed, and the weights were subtracted from the initial weight (50g). The weight loss after drying was recorded as the moisture content of the meat sample.
Moisture content = Fresh meat weight – Dried meat weight
Water holding capacity
The water holding capacity (WHC) of the meat was determined by placing 1 g of breast meat sample between two filter papers (9 cm Whatman No 1) and then enclosed in between two 10.2 x 10.2 cm plexi glasses and pressed between two jaws of a vice with a 35 kg/cm3 force for 1 minute. The areas of free water and pressed meat samples were measured using grid method, while percent free water capacity was calculated based on weight and moisture content. Water holding capacity was calculated as follows:
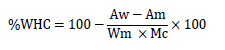
Where:
% WHC = Percentage water holding capacity
Aw = Area of water released from meat samples (cm2)
Am = Area of meat samples (cm2)
Wm = Weight of meat (g)
Mc = Moisture content of meat samples (%)
Cooking loss
50g samples of breast muscle samples were wrapped in an airtight polythene bag, labeled accordingly and immediately cooked in water bath to a temperature of 70 °C for 15 minutes. Thereafter, the water was released after cooking and cooling was effected and the weight of the cooked meat chops was measured to obtain the cooking loss. The percentage cooking loss was expressed as:
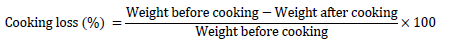
pH
The pH of breast meat was determined using a pH meter. OAKIAN Digital pH meter (Pc 2700) was calibrated using certified buffer solution and an electrode was used to touch the surface of the meat samples. The readings were noted for each meat samples and recorded to the nearest 0.01 unit.
Lipid profile determination
The breast muscle samples were chopped and refrigerated at -20 °C. The samples were later blended by feeding them into a laboratory blender (Waring 3061-01). The homogenized tissues were then received and centrifuged by separating the supernatant from the residue, which was later used for total cholesterol, high density lipoprotein (HDL) cholesterol and low density lipoprotein (LDL) cholesterol using fortress diagnostics analysis. The lipid profile is precipitated by the addition of heparin at their isoelectric point (pH 5.04). The total cholesterol was measured enzymatically in a series of coupled reactions that hydrolyze cholesteryl esters and oxidize the 3-OH group of cholesterol. The by-produced H2O2 was measured quantitatively in a peroxidase catalyzed reaction that produces a color. Absorbance was measured at 500 nm. The color intensity was proportional to total cholesterol concentration. The HDL and LDL remain in the supernatant and were then determined through enzymatic (colorimetric) methods. LDL cholesterol was then calculated from the values measured for total cholesterol and HDL cholesterol according to the relationship:
(LDL cholesterol) = Total cholesterol – HDL cholesterol – VLDL
VLDL = Triglycerides content × 0.2
Statistical analysis
Data was subjected to statistical analyses through one-way analysis of variance (ANOVA) using the General Linear Model Procedure of the SAS (SAS Institute Inc [25]) software. Significant differences among means were distinguished by Tukey’s Honest Significant Difference (HSD) procedure. Differences at 5% level of probability were considered significant.
Results
Physico-chemical characteristics of broiler meat
At the 28th day, the moisture content of broiler meat in the T5 treatment group was significantly higher compared to the T1 and T2 groups but did not differ significantly from the T3 and T4 groups. The water-holding capacity of broiler meat in T2 was significantly greater than in T1, T3, and T5 groups, while being comparable to that in of T4 group. No significant differences were observed in the percentage of cooking loss or the pH of the broiler meat among the dietary treatments at this age (Table 3).
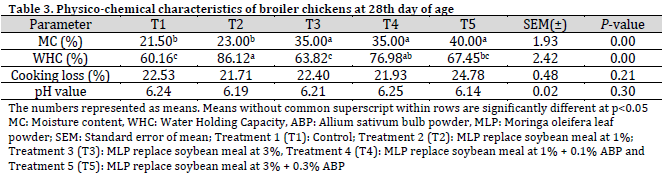
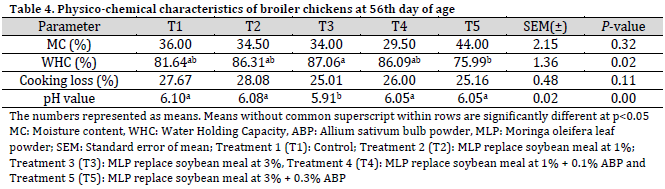
At 56 days of age, the moisture content of the meat did not show significant differences among the treatment groups, albeit T5 treatment exhibited the highest moisture content value at 44%. The water-holding capacity of broiler meat across the dietary treatments (T2, T3, T4, and T5) was comparable to that of the control group. Notably, the pH of broiler meat in the T3 group was significantly reduced in comparison to the T1 group and the other treatment groups (Table 4).
Lipid profile of broiler meat
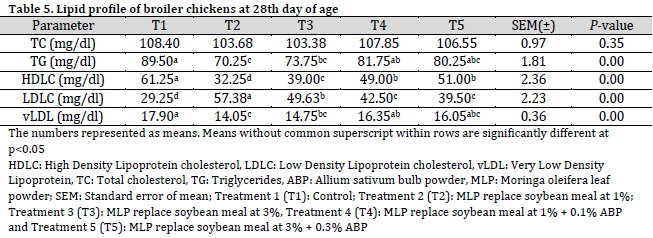
The total cholesterol levels were not significantly influenced by the dietary treatments after 28 days. However, triglycerides and very low-density lipoprotein (vLDL) cholesterol levels were significantly decreased in the treatment groups compared to the control group (T1). On the other hand, high-density lipoprotein (HDL) levels in the control group (T1) were significantly higher than those in the dietary treatment groups (T2, T3, T4, and T5). Conversely, low-density lipoprotein (LDL) cholesterol levels were significantly lower in the control group (T1) compared to MLP and ABP supplemented diets (Table 5).
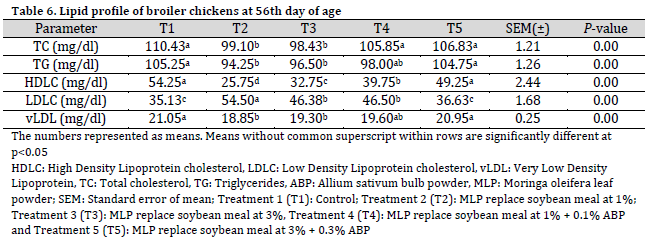
At 56 days of age, total cholesterol, triglycerides, and vLDL levels in T2 and T3 groups were significantly lower compared to those in T1, T4, and T5. HDL cholesterol levels in the control group (T1) remained significantly higher than that in T2, T3, and T4, but were comparable to T5. LDL cholesterol levels in the control group (T1) were significantly lower than those in T2, T3, and T4, but were similar to T5 (Table 6).
Discussion
This study examined the effects of dietary supplementation with Moringa oleifera leaf powder (MLP) and Allium sativum bulb powder (ABP) on the physicochemical characteristics and lipid profile of broiler chickens. moisture content and water-holding capacity of broiler meat were significantly enhanced across the dietary treatments after 28 days. These results suggest that the inclusion of antioxidant-rich Moringa oleifera leaf powder and Allium sativum bulb powder in the diet could be an effective strategy for improving the physicochemical properties of broiler meat. Dietary manipulation remains a key approach for enhancing meat quality in poultry production [3]. A notable reduction in the pH of broiler meat was observed in the T3 group at the 56th day, approaching the typical ultimate pH range for broiler meat (5.70-5.76) [26]. Other studies reported no significant effects were observed on the breast meat pH of broiler chickens fed Moringa oleifera leaf meal at inclusion rates of 1%, 3%, and 5% of dry matter intake [27], which agrees with the current findings except for the aforementioned observation (T3 at the 56th day).
At 56 days of age, improvements in total cholesterol, triglycerides, and very low-density lipoprotein (vLDL) cholesterol were observed in birds fed the dietary treatments, aligning with previous reports of significant reductions in total cholesterol in broiler chickens fed a garlic-supplemented diet [28]. These effects are likely attributable to the hypocholesterolemic influence of Moringa oleifera and Allium sativum, which may suppress the hepatic activities of lipogenic and cholesterogenic enzymes. LDL cholesterol levels increased, while HDL cholesterol levels decreased in the dietary treatments compared to the control diet. However, the HDL and LDL levels in the T5 group were comparable to those in the control diet at 56 days of age. This finding contrasts with the results reported by [5], who observed a gradual increase in serum HDL concentration and a reduction in serum LDL concentration in broilers fed a Moringa oleifera-supplemented diet. The general reduction in HDL and increase in LDL values across treatments may be linked to physiological changes, such as the oxidative status of the birds. Factors such as suboptimal diet, certain medications, and exposure to environmental stressors (e.g., radiation, toxins, air pollution, and intense sunlight) can lead to these deviations, creating an imbalance between free radicals and antioxidants. However, T5 treatment led to a comparable lipid profile to that of control, where higher HDL (often known as good cholesterol) and lower LDL (often known as bad cholesterol) levels were obtained, which is preferred from a dietary prospective. Another study found that total cholesterol, triglycerides, LDL, and vLDL levels decreased in broilers fed diets supplemented with Allium sativum [28].
Conclusion
The study concluded that the dietary inclusion of Moringa oleifera leaf powder and Allium sativum bulb powder improved the physicochemical characteristics of broiler chickens, notably at 28 days of age. This improvement could benefit producers who slaughter their birds between the 28th and 35th days. Despite the known antioxidant and hypolipidemic properties of Moringa oleifera, the lipid profile parameters, specifically HDL and LDL cholesterol, did not consistently improve. However, higher garlic powder supplementation combined with moringa leaf powder (T5) resulted in a comparable lipid profile to that of control. Therefore, including garlic powder in combination with moringa leaf powder (T5) can enhance some of the cooking characteristics of broiler meat (such as moisture content) while maintaining a dietary healthier lipid profile.
References
| 1 | Agbetuyi OA, Ekeocha AH, Aganga AA, Apata TG. The use of Moringa oleifera and Allium sativum, and funding of poultry production in Ekiti State, Nigeria. J. Agric. Food Environ. 2023;10(3):11-23. |
| 2 | Hajati H, Hassanabadi A, Ahmadian F. Application of Medicinal Plants in Poultry Nutrition. J. medicinal plants by-products. 2014;3(1):1-12. DOI |
| 3 | Cheng Y, Chen Y, Li J, Qu H, Zhao Y, Wen C, Zhou Y. Dietary β-Sitosterol improves growth performance, meat quality, antioxidant status, and mitochondrial biogenesis of breast muscle in broilers. Animals. 2019;9(3):71. DOI |
| 4 | Vinus, Dalal R, Sheoran N, Maan NS, Tewatia BS. Potential benefits of herbal supplement in poultry feed: A review. The Pharma. Inno. J. 2018;7(6):651-6. |
| 5 | Sugiharto S, Isroli I, Yudiarti T, Widiastuti E, Wahyuni HI, Sartono TA. Performance, physiological and microbiological responses of broiler chicks to Moringa oleifera leaf powder, garlic powder or their combination. Livest. Res. Rural Dev. 2018;30(12):209. |
| 6 | Agbetuyi OA, Ekeocha AH, Aganga AA. Organoleptic parameters, tibia bone growth and mineral retention of broiler chicken fed Moringa oleifera and Allium sativum. Slovak J. Anim. Sci. 2023b;56(3):3-12. DOI |
| 7 | Agbetuyi OA, Oloruntola OD. Effects on performance of broiler chickens of feeding Shea butter as replacement for vegetable oil in their diet. Livest. Res. Rural Dev. 2020;32(6). |
| 8 | Nouman W, Basra SMA, Siddiqui MT, Farooq H, Zubair M, Gull T. Biomass production and nutritional quality of Moringa oleifera as field crop. Turk. J. Agri. For. 2013;37(4):410-9. DOI |
| 9 | Ayegba C, Makinde O, Obigwa P, Orijajogun J. Effect of drying temperature on nutritional content of Moringa oleifera leaves. World J. Food Sci. and Tech. 2017;1(3):93-6. |
| 10 | Agbetuyi OA, Ekeocha AH, Aganga AA. Assessing the impact of moringa and garlic supplementation on egg production and quality in laying hens. Dysona Appl. Sci. 2024;5(2):41-51 DOI |
| 11 | Saini RK, Shetty NP, Prakash M, Giridhar P. Effect of dehydration methods on retention of carotenoids, tocopherols, ascorbic acid and antioxidant activity in Moringa oleifera leaves and preparation of a RTE product. J. Food Sci. Technol. 2014;51:2176-82. DOI |
| 12 | Gandji K, Chadare FJ, Idohou R, Salako VK, Assogbadjo AE, GleleKakai RL. Status and Utilization of Moringa oleifera Lam: A review. Afr. Crop Sci. J. 2018;26(1):137-56. DOI |
| 13 | Rehman HF, Zaneb H, Masood S, Yousaf MS, Ashraf S, Khan I, Shah M, Khilji MS, Rehman H. Effect of Moringa Oleifera Leaf Powder Supplementation on Pectoral Muscle Quality and Morphometric Characteristics of Tibia Bone in Broiler Chicken. Braz. J. Poultry Sci. 2018;20(4):817-24. DOI |
| 14 | Pandey A, Pradheep K, Gupta R, Nayar ER, Bhandari DC. Drumstick tree’ (Moringa oleifera Lam.): A multipurpose potential species in India. Genet. Resour. Crop Evol. 2011;58(3):453-60. DOI |
| 15 | Pucava N, Stanacev V, Glamocic D, Levic J, Peric L, Milic D. Beneficial effects of phytoadditives in broiler nutrition. World’s Poult. Sci. J. 2013;69:27-34. DOI |
| 16 | Uhegbu FO, Iweala EEJ, Kanu I. Studies on the chemical and antinutritional content of some Nigerian spices. Int. J. Nutr. Metab. 2011;3(6):72-6. |
| 17 | Hanieh H, Narabara K, Piao M, Gerile C, Abe A, Kondo Y. Modulatory effects of two levels of dietary Alliums on immune responses. Anim. Sci. J.2010;81:673-80. DOI |
| 18 | Mansoub NH. Comparative effects of using garlic as probiotics on performance and serum composition of broiler chickens. Ann. Biol. Res. 2011;2:486-90. |
| 19 | Rahmatnejad E, Roshanfekr H. Evaluation of effects of several non-antibiotics additives on growth performance of broiler chickens. J. Vet. Adv. 2009;8:1757-60. |
| 20 | Stanacev V, Glamocic D, Milosevic N, Peric L, Puvaca N, Stanacev V, Milic D, Plavsa N. Influence of garlic (Allium sativum) and copper as phytoadditives in the feed on the content of cholesterol in the tissue of the chickens. J. Med. Plants Res. 2012;6(14):2816-9. |
| 21 | Zekic V, Puvaca N, Milic D, Beukovic M, Glamocic D, Vukelic N, Zekic S. Effect of garlic powder in broiler chicken nutrition: Emphasis on production economic efficiency costs and chicken meat quality. Custos Agronegocio. 2014;10(2):86-98. |
| 22 | Kamruzzaman AMS, Khandaker ZH. Effects of feeding garlic powder on growth performance and meat quality of Broiler.Bang. J. Anim. Sci. 2016;45(2):79-83. DOI |
| 23 | Eleroglu H, Yildirim A, Nursel DI, Cekeroglu A, Duman M. Comparison of meat quality and fatty acid profile in slow-growing chicken genotypes fed diets supplemented with Origanum vulgare or Melissa officinals leaves under the organic system. Ital. J. Anim. Sci. 2013;12(3):395-403. DOI |
| 24 | Zhang C, Yang L, Zhao X, Chen X, Wang L, Geng Z. Effect of dietary resveratrol supplementation on meat quality, muscle antioxidative capacity and mitochondrial biogenesis of broilers. J. Sci. Food Agric. 2017;98(3):1216–21. DOI |
| 25 | SAS Institute. User’s guide: statistics. Version 9.2; SAS Institute, Inc.: Cary, NC, USA 26. 2013. |
| 26 | Łukasiewicz M, Pietrzak D, Michalczuk M, Mroczek J, Niemiec J, Chmiel M. Effect of selected feed additives used in coccidiosis prophylaxis on growth performance and carcass and meat quality of fast-growing Hubbard Flex chickens. Eur. Poultry. Sci. 2014;78:1612. DOI |
| 27 | Nkukwana TT, Muchenje V, Masika PJ, Hoffman LC, Pieterse E, Dzama K. Proximate composition and variation in colour, drip loss and pH of breast meat from broilers supplemented with Moringa oleifera leaf meal over time. Anim. Prod. Sci. 2015;56(7):1208-16. DOI |
| 28 | Issa KJ, Omar JMA. Effect of garlic powder on performance and lipid profile of broilers. Open J. Anim. Sci. 2012;2(2):62-8. DOI |
Cite this article:
Agbetuyi, O., Ekeocha, A., Aganga, A. Modulation of physico-chemical traits and lipid profile in broiler meat through moringa and garlic supplementation. DYSONA – Applied Science, 2024;5(2): 52-61. doi: 10.30493/das.2024.451989
