Abdelhak Rhouma 1*; Lobna Hajji-Hedfi 1; Pravin Babasaheb Khaire 2
1, Regional Centre of Agricultural Research of Sidi Bouzid, CRRA, Gafsa Road Km 6, B.P. 357, 9100, Sidi Bouzid, Tunisia
2, Department of Plant Pathology and Microbiology, Mahatma Phule Krishi Vidyapeeth, Rahuri 413722 Maharashtra, India
E-mail:
abdelhak.rhouma@gmail.com
Received: 08/08/2024
Acceptance: 21/09/2024
Available Online: 23/09/2024
Published: 01/01/2025

Manuscript link
http://dx.doi.org/10.30493/DAS.2024.472342
Abstract
Botryosphaeriaceae fungi are causal agents of panicle and shoot blight, a significant disease impacting pistachio and other nut crops worldwide. Initially described as Camarosporium pistaciae in Greece and later as Botryosphaeria dothidea in California, the disease is characterized by canker formation, shoot dieback, and fruit lesions. Symptoms include blackened shoots, necrotic leaf lesions, and fruit covered in pycnidia (fungal fruiting structures). Economic losses are substantial, with yield reductions in California reaching 40-100% during outbreaks. Several factors contribute to disease severity, including weather patterns, agricultural practices, and climate change. High-density planting and mechanical harvesting practices increase infection risk by creating wounds that serve as entry points for the pathogen. Extreme weather events like El Niño can worsen disease severity by promoting fungal growth and spore dispersal. Botryosphaeriaceae pathogens survive in infected tissues and release spores under wet conditions. The disease cycle involves initial infections by pycnidiospores dispersed by rain and secondary spread within the tree. Tools, machinery, and insect vectors can further facilitate pathogen dissemination. Effective management necessitates a multifaceted approach encompassing optimized pruning practices, weather monitoring for informed fungicide application, integrating cultural practices, chemical treatments, and biological control to minimize the impact of Botryosphaeriaceae on pistachio and other nut crops. This review provides a thorough analysis of Botryosphaeria panicle and shoot blight disease in nut crops. It dissects the causative Botryosphaeriaceae fungi through examination of their taxonomy and morphology. Besides, this review explores disease development, detailing signs and symptoms, followed by an analysis of the infection process and pathogenicity. Finally, it emphasizes integrated disease management strategies, combining chemical, cultural, and biological controls, while discussing the potential for long-term management through host plant resistance.
Keywords: Botryosphaeriaceae, Shoot blight, Neofusicoccum mediterraneum, Botryosphaeria dothidea, Pistachio, Olive
Introduction
A fungus known as Botryosphaeriaceae (Botryosphaeriales, Ascomycetes) is found all over the world and is responsible for a variety of diseases on woody plants [1]. Certain species of Botryosphaeriaceae can infect buds, fruits, petioles, rachises, midribs of leaflets, shoots, and branches [2][3]. This can lead to the death of fruit panicles, hull and kernel decay, dark shell staining, and blight of panicles and shoots on pistachio (Pistacia vera). The pistachio industry in California, USA was considered severely threatened by the pistachio panicle and shoot blight caused by species of Botryosphaeriaceae, particularly Neofusicoccum mediterraneum [2-4], until recently when efficient disease management was developed [5]. There have been reports of several phytopathogenic species of Botryosphaeriaceae in areas where P. vera trees are planted. There have been reports of Botryosphaeria dothidea, Neofusicoccum parvum, and N. australe on P. vera in Australia [4][6], B. dothidea and N. parvum in Greece [4][7], B. dothidea and Diplodiaseriata in South Africa [8], N. australe in Spain [9], and Lasiodiplodia Theobromae and N. mediterraneum in Arizona, USA [4][7].
Eight species of the Botryosphaeriaceae family have been identified on P. vera in California: B. dothidea, Diplodia seriata, Dothiorella iberica, Dothiorella sarmentorum, Lasiodiplodia citricola, Lasiodiplodia gilanensis, Neofusicoccum mediterraneum, and Neofusicoccum vitifusiforme [4][7]. According to pathogenicity test results, the species most harmful to the two main cultivars of P. vera produced in California-the male Peters and the female Kerman-are L. citricola and L. gilanensis, followed by N. mediuterraneum [4].
This review focuses on Botryosphaeria panicle and shoot blight disease, a major concern for nut crops. It delves into the causal agent, Botryosphaeriaceae fungi, by examining their taxonomic classification and physical characteristics (morphology). The review then explores the disease cycle, detailing the visible signs and symptoms exhibited by infected plants. This is followed by a scientific analysis of how the infection progresses and how the fungus establishes itself within the host (pathogenicity). Finally, the review emphasizes integrated disease management strategies. This combines the use of fungicides (chemical control), cultural practices that reduce disease spread (cultural control), and the application of beneficial organisms to combat the pathogen (biological control). The potential for developing nut crop varieties resistant to the disease through breeding programs is also discussed.
Symptomatology, economic importance, and etiology
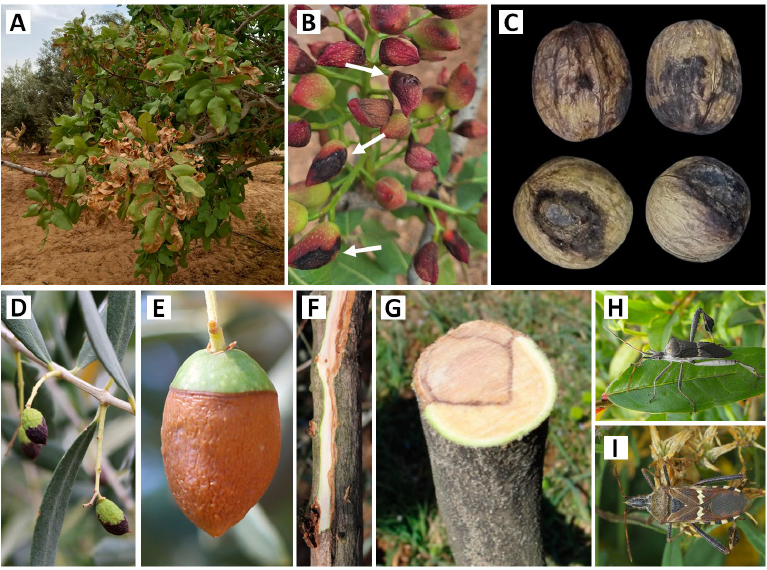
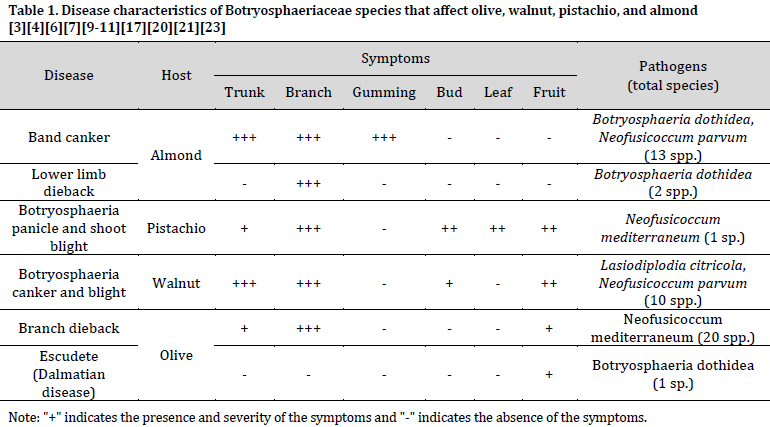
Panicle and shoot blight caused by Botryosphaeriaceae pathogens is a major disease of nut and olive trees [6][10]. The disease was first identified as a new species of Camarosporium (C. pistaciae) in Greece [11] and later as B. dothidea in California [3]. The pathogen infects various parts of the tree including trunks, branches, shoots, leaves, and fruits [6][12][13] (Fig. 1). Botryosphaeriaceae species infect young trees near the graft union, leading to stem cankers and potentially causing seedling death [4][8]. The infection causes cankers on trunks and branches, with a distinguished V-shaped infection zone appearing in the cross-section of infected trunks and branches (Fig. 1 G). The fungus can invade the xylem and trees can respond by healing around cankers to prevent further spread [14]. While cankers may not necessarily kill branches or the entire tree, the disease causes significant damage to the new growths. Shoots infected postharvest through scars on buds, leaves, and panicles develop a necrotic state, characterized by blackening and eventual death (Table 1) [6][15]. Male bud infection might not affect yield due to abundant pollen production. Still, female flower bud infection can lead to panicle collapse, which is considered the most devastating aspect of the disease [16][17]. Furthermore, the pathogen can infect leaf petioles and leaflets, causing blight and defoliation [16][17]. Leaf infection symptoms vary depending on the season, with necrotic black lesions in spring and brown centers with chlorotic margins in summer (Fig. 1 A) [18][19]. In pistachio and other nuts, infection appears as rounded black lesions covering large areas or even the entire fruit (Fig. 1 B and C). The disease reach extends beyond established orchards, causing significant economic losses in nurseries as well.
Botryosphaeriaceae Panicle and shoot blight poses a significant economic threat to pistachio production worldwide [6][9][20-22]. Epidemic years can result in devastating losses, with historical reports from California documenting yield reductions between 40% and 100% in the northern counties during the late 1980s [3][13]. Particularly, wet years, such as the 1998 El Niño event, exacerbate the disease impact due to favorable conditions for fungal growth and potential limitations of fungicide efficacy [3]. This was exemplified in California in 1998, with a “Bot year” where the disease caused an estimated 9000 tons in production losses [3]. In the United States, wet springs, exemplified by the event in 1995, can also trigger multi-year epidemics, disrupting upward production trends. The global presence of pistachio panicle and shoot blight has been confirmed in major pistachio-producing countries including Australia, Italy, Iran, Spain, and South Africa [6][9][20][21]. Furthermore, the diversity of the causal agent has expanded, with the number of identified Botryosphaeriaceae species associated with pistachio trees reaching 15. These species include Botryosphaeria dothidea, Diplodia seriata, Dothiorella iberica, and various others [4][7][9][11][23][24].
The past decade has witnessed a concerning rise in both the incidence and severity of diseases caused by Botryosphaeriaceae fungi. This concerning trend can be attributed to several contributing factors [4][7][23]. Agricultural practices in California and the Mediterranean Basin contribute to increased risk of Botryosphaeriaceaefungal diseases in nuts and olives. Firstly, these regions tend to establish orchards in humid locations near water sources, creating ideal environments for fungal growth. Additionally, these areas often harbor a variety of wild plant hosts susceptible to Botryosphaeriaceae spp., which act as a constant source of inoculum for nearby orchards [7][25][26]. Secondly, rising land values and economic incentives have driven a shift towards high-density planting systems like hedgerows. In these systems, trees are planted close together, leading to the development of dense canopies [27]. These high densities create microclimates with extended periods of leaf wetness, a critical factor for fungal disease development. In conclusion, the combination of humid environments with nearby wild hosts and the adoption of high-density planting systems creates a favorable scenario for Botryosphaeriaceae fungal disease outbreaks [28]. Intensive cropping systems for nut and olive production elevate the risk of Botryosphaeriaceae fungal diseases through several mechanisms associated with harvesting and pruning practices. Mechanical harvesting methods, including trunk shakers and canopy-contact harvesters used in hedgerows and traditional orchards, inflict wounds on the trees (e.g., bark loss, branch breakage) that act as infection points for these pathogens. Similarly, manual pruning practices can create wounds susceptible to fungal colonization [13][29][30]. The frequency and intensity of pruning further influence disease risk, with some regions like California and Turkey adopting a minimal pruning approach to maximize yield, while others like the Mediterranean Basin rely on more frequent hand pruning. Regardless of the method, wounds become potential infection sites if inoculum is present and environmental conditions favor fungal growth [3][31]. Furthermore, harvesting and pruning tools can inadvertently transfer pathogen inoculum within and between orchards, facilitating disease spread. Therefore, these intensive cropping systems create a scenario conducive to Botryosphaeriaceae infection due to increased wounding from harvesting and pruning, potential for inoculum transfer, and possibly altered microclimates within the dense canopies.
Beyond the influence of agricultural practices, several other factors contribute to the vulnerability of nut and olive trees to Botryosphaeriaceae diseases. Extreme weather events, such as El Niño and drought periods, can potentially impact populations of other fungal pathogens (e.g., Phomopsis spp.) or insect pests (e.g., walnut scale, stinkbugs). Increased pressure from these co-existing organisms could weaken trees and make them more susceptible to Botryosphaeriaceae outbreaks [26][32][33]. Studies have shown a correlation between the presence of pests like scales and the susceptibility of host trees to Botryosphaeriaceae infection [34]. Furthermore, large Hemipteran insects (Fig. 1 H and I) can act as vectors for these pathogens by spreading inoculum while feeding and creating wounds on the trees [26]. Management practices can also influence disease risk. In olive orchards, the traditional burning of pruning residues was thought to reduce fungal inoculum, while the current practice of chipping and mulching prunings on the ground may lead to increased inoculum pressure. However, research in walnuts suggests that composting debris contaminated with fungal pathogens like Botryosphaeriaceae or Diaporthaceae may not pose a threat. Studies indicate high composting temperatures effectively eliminate these pathogens, making it potentially safe to return composted material to the orchard [26][34].
Outbreaks of Botryosphaeriaceae-induced diseases are most likely during seasons with excessive rainfall [18][19]. This increased risk stems from two key factors: water-dispersed conidia facilitating primary infections, and the potential for these infections to remain latent through fall and winter, emerging as disease symptoms the following spring and summer [3]. Consequently, periods of high rainfall lead not only to the initial disease outbreak but also contribute to inoculum buildup for future years. Furthermore, the irregular occurrence of El Niño events (every 2-7 years) can create cyclical epidemics, particularly if management practices are not consistently implemented [35-37]. Additionally, drought years following periods of high rainfall can exacerbate disease severity. Water stress compromises host defenses, making trees more susceptible to Botryosphaeriaceae infection. Studies have shown, for instance, that water-stressed pistachio trees are more susceptible to Neof. mediterraneum compared to well-watered trees [38]. Similarly, extreme cold events can also increase disease risk by causing cracks and wounds in trees, creating entry points for pathogens. An example of this is the outbreak of Diplodia seriata, typically a weak pathogen, associated with dieback of young olive trees that had been exposed to exceptionally low winter temperatures in Croatia. These findings highlighted the complex interplay between weather events, inoculum pressure, and host susceptibility in influencing the severity of Botryosphaeriaceae diseases [39] (Table 1).
Climate change predictions pose a potential threat to nut and olive crops by increasing the severity of Botryosphaeriaceae diseases. Many climate models predict a rise in extreme weather events, including increased summer temperatures and water stress in the Mediterranean Basin, a region already conducive to these fungal diseases. While California may experience increased precipitation alongside rising temperatures, both scenarios (increased water stress and increased rainfall) can be in favor of Botryosphaeriaceae pathogens [40].
Disease cycle
The perennial nature of almond, pistachio, olive, and walnut crops, coupled with regional climates, shapes the disease cycles of Botryosphaeriaceae fungi. Mediterranean climates, typical for many of these crops, are characterized by dry summers and cool, wet winters. Asexual spores (pycnidiospores) serve as the primary inoculum source, with sexual spores (ascospores) playing a minor role. Interestingly, the risk of epidemics can be significantly influenced by insects. These insects may act as vectors for the pathogen or increase plant susceptibility through feeding damage. Furthermore, Botryosphaeriaceae species exhibit a saprophytic phase, colonizing both living tree bark and dead plant debris on the ground. This ability to survive on dead tissue contributes to the persistence of these pathogens in orchards
Pathogen survival
Botryosphaeriaceae fungi exhibit various mechanisms for overwintering and oversummering. These pathogens primarily survive as pycnidia or pseudothecia, which develop within protective structures called stromata. The thickness of the stroma wall varies depending on the species, with thicker walls offering greater protection from environmental degradation. Additionally, infected epidermal tissues can further shield the stromata [41] and fruiting bodies [42] from harsh weather conditions and fungicide sprays. Botryosphaeriaceae can persist in various infected tissues including cankered trunks, dead branches, blighted shoots, and fruit [13][43]. For annual plant tissues like leaves or fruit on deciduous trees, the pathogen survives within infected structures on the tree or fallen to the ground [16][17][25]. However, saprophytic microbes in the soil can reduce pathogen viability in fallen debris [26]. Interestingly, survival rates are significantly lower when cankered branches are chipped [16] [17][44]. Pistachio trees further enhance pathogen survival by retaining infected rachises and fruits for longer periods compared to healthy tissues [13]. Studies have shown viable pycnidiospores persisting in blighted pistachio rachises and cankers for up to 2 and 6 years, respectively [13]. The presence of fruiting structures also varies by host. While readily observed in cankered branches of pistachio, olive, and walnut [3][16][17][29][44], they are rare in almond shoots or spurs. However, exceptions exist, with reports of pseudothecia alongside pycnidia in bark of severely cankered almond trunks, stumps of Botryosphaeriaceae-killed almond trees [45], and blighted walnut shoots. Furthermore, Botryosphaeriaceae can overwinter in infected and blighted buds of pistachio and walnut trees. These diverse survival strategies across different tissues and hosts contribute to the persistence and challenge of managing Botryosphaeriaceae diseases [3][14][26].
For Botryosphaeriaceae fungi, inoculum originates primarily from two sources: neighboring trees and the environment. In newly planted orchards with healthy nursery trees, spores arrive from surrounding established trees within the same field or from nearby alternative hosts that may also be susceptible. However, within established orchards with ongoing infections, new infections are largely driven by internal sources of inoculum. Most Botryosphaeriaceae species appear to have poor survival rates in soil environments. This suggests that these pathogens rely more heavily on infected plant material within the orchard for survival and disease spread [3][14][26].
Epidemiology
Species of Botryosphaeriaceae display monocyclic or oligocyclic life cycles, resulting in polyetic epidemics [46]. Monocyclic pathogens complete a singular disease cycle, or a segment thereof, within one growing season. Depending on meteorological conditions, certain species may operate as oligocyclic pathogens, completing two or three cycles per season [47]. In both scenarios, the progression of the disease and the intensity of symptoms are significantly affected by the initial inoculum pressure [48]. Polyetic epidemics are defined by the inoculum generated in one season serving as the primary inoculum for the subsequent season [49]. As a result, these diseases may demonstrate prolonged epidemic durations lasting several years [50]. This underscores the significance of regulating inoculum sources and disrupting disease cycles to mitigate the effects of Botryosphaeriaceae pathogens on vulnerable crops [51][52].
Disease control
Effective control of Botryosphaeriaceae diseases in perennial crops necessitates an integrated approach [50]. Orchard management practices that enhance air circulation and exposure to sunlight within the tree canopy are essential for mitigating infection risk and disease severity. [53]. This can be achieved by maintaining appropriate tree density and implementing canopy management strategies. Pruning practices should prioritize the removal of dead or infected tissues harboring fungal fruiting bodies, thereby minimizing inoculum sources within the orchard. Supplementary sanitation protocols involve the removal and chipping or incineration of pruning material, as well as eliminating stumps of trees removed due to Botryosphaeriaceae-induced diseases like almond band canker [54]. These sanitation practices combined with other potential control methods like fungicides can contribute to a comprehensive integrated disease management strategy for Botryosphaeriaceae pathogens [34][55].
Proper irrigation management plays a crucial role in minimizing Botryosphaeriaceae disease development. Studies in pistachio orchards by [53] and [56] showed a significant reduction in blighted cluster incidence (from 92% to 21%) when using low-trajectory sprinklers that minimize wetting of the trunk and canopy compared to high-trajectory sprinklers [53]. This highlighted the importance of irrigation techniques that avoid excessive wetting of plant parts susceptible to infection. Similarly, for almond trees affected by Leathery Leaf Disease and Bud Death, avoiding over-irrigation during spring is crucial. Excessive spring moisture can create favorable conditions for Botryosphaeriaceae growth and potentially exacerbate disease symptoms [56]. These findings emphasize the need for irrigation practices that balance water needs with disease control strategies for Botryosphaeriaceae pathogens [57-59].
While complete resistance to Botryosphaeriaceaespecies is not known among almond, pistachio, or walnut cultivars, varying levels of susceptibility have been documented [60]. For instance, in pistachios, the common Kerman and Lost Hills cultivars exhibit branch susceptibility but possess highly resistant fruit [34]. The most significant cultivar variations are observed in olives. Olive cultivar Gordal Sevillana displays pronounced susceptibility to Botryosphaeria dothidea under both artificial and natural infection conditions [60]. Furthermore, this cultivar shows increased susceptibility to escudete disease compared to Hojiblanca and Picual cultivars under field conditions. Similar trends of cultivar-dependent fruit susceptibility to escudete have been reported in Spain and Montenegro. However, it is important to note that cultivar attractiveness to olive fly may also play a role in the observed percentages of diseased fruit, as olive fly infestation can create entry points for the escudete-causing Botryosphaeria dothidea fungus [16][17][61-63]. More efforts should be concentrated on understanding the genetic factors controlling the susceptibility and resistance toBotryosphaeriaceae and to incorporate this acquired knowledge into breeding programs of fruit trees [10][63].
While some success has been achieved with synthetic fungicides, management of Botryosphaeriaceae diseases relies heavily on preventative measures and alternative control strategies. A study by [64-66] found that the Paenibacillus lentimorbus CBCA-2 is a promising biological control agent for Botryosphaeria dothidea. Trials using Trichoderma strains for pistachio crop protection have generally been unsuccessful [67]. However, further investigation into the use of other biocontrol agents, such as yeast and bacteria, is warranted. In contrast, various classes of synthetic fungicides, including premixes of strobilurins and carboxamides, have demonstrated excellent efficacy against pistachio panicle and shoot blight under field conditions [67][53]. For this disease, optimal control is achieved with spray applications of fungicides at early bloom followed by another application in early summer [3]. Growers can leverage advisories from local weather monitoring programs to ensure accurate and effective spray timing in pistachio and walnut orchards [5]. For olive crops, fungicide applications are most effective after harvest to protect wounds caused by harvesting equipment and at the end of winter, when mature ascospores are prevalent [34][44][56]. In the case of escudete disease, controlling the olive fly population remains the most efficient management strategy for the fungal pathogen Botryosphaeria dothidea. Interestingly, Prolasioptera berlesiana, a predator of olive fly eggs, was recently reported as the main factor in B. dothidea pathogen spreading, whereas the fruit fly merely acts as a conduit in the transmission chain [62]. Consequently, a deeper comprehension of control methodologies is essential to ensure the effective management of Botryosphaeriaceae diseases.
Conclusion
Diseases caused by Botryosphaeriaceae primarily result from agricultural practices that foster optimal conditions for fungal proliferation. The severity of the disease is intensified by extreme weather events and management practices. Effective disease management necessitates integrated strategies, encompassing appropriate irrigation, optimal tree spacing, pruning, and sanitation. The genetic mechanisms underlying disease susceptibility remain unidentified, necessitating further investigation into disease resistance and plant breeding for enhanced disease resilience. An in-depth comprehension of control methodologies is crucial for the effective management of Botryosphaeriaceae diseases. Consequently, additional research into biocontrol agents, including yeast and bacteria, is necessary.
References
- Slippers B, Wingfield MJ. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol. Rev. 2007;21:90-106. DOI
- Michailides TJ, Morgan DP. Effects of temperature and wetness duration on infection of pistachio by Botryosphaeria dothidea and management of disease by reducing duration of irrigation. Phytopathology. 1992;82:1399-406. DOI
- Michailides T, Morgan DP. Panicle and Shoot Blight of Pistachio: A Major Threat to the California Pistachio Industry. APSnet Features. 2004;2004:1-15. DOI
- Chen SF, Morgan DP, Hasey JK, Anderson K, Michailides TJ. Phylogeny, morphology, distribution, and pathogenicity of Botryosphaeriaceae and Diaporthaceae from English walnut in California. Plant Dis. 2014;98:636-52. DOI
- Morgan DP, Driever GF, Felts D, Krueger WH, Michailides TJ. Evaluation of two disease-warning systems for Botryosphaeria panicle and shoot blight of California pistachio and efficient control based on early- season sprays. Plant Dis. 2009;93:1175-81. DOI
- Wunderlich N, Costa S, Tpoi R, Ash G. first report of Botryosphaeria dothidea causing shoot blight and cankers of pistachio in Australia. Australas. Plant Dis. Notes. 2012;7:47-9. DOI
- Inderbitzin P, Bostock RM, Trouillas FP, Michailides TJ. A six-locus phylogeny reveals high species diversity in Botryosphaeriaceae from California almond. Mycologia. 2010;102:1350-68. DOI
- Swart WJ, Botes WM. First report of stem canker caused by Botryosphaeria obtusa on pistachio. Plant Dis. 1995;79:1036-8. DOI
- Armengol J, Gramaje D, Perez-Sierra A, Landeras E, Alzugaray R, Luque J, Martos S. First report of canker disease caused by Neofusicoccum australe on eucalyptus and pistachio in Spain. Plant Dis. 2008;92:980. DOI
- Linaldeddu BT, Rossetto G, Maddau L, Vatrano T, Bregant C. Diversity and pathogenicity of Botryosphaeriaceae and Phytophthora species associated with emerging olive diseases in Italy. Agriculture. 2023;7;13(8):1575. DOI
- Chen S, Li G, Liu F, Michailides T. Novel species of botryosphaeriaceae associated with shoot blight of pistachio. Mycologia. 2015;107:780-92. DOI
- Petrović E, Vrandečić K, Belušić Vozila A, Ćosić J, Godena S. Diversity and Pathogenicity of Botryosphaeriaceae Species Isolated from Olives in Istria, Croatia, and Evaluation of Varietal Resistance. Plants. 2024;13(13):1813. DOI
- Michailides TJ. Pathogenicity, distribution, sources of inoculum, and infection courts of Botryosphaeria dothidea on pistachio. Phytopathology. 1991;81:566-73. DOI
- Ntahimpera N, Driever GF, Felts DG, Morgan DP, Michailides TJ. Dynamics and pattern of latent infection caused by Botryosphaeria dothidea on pistachio buds. Plant Dis. 2002;86:282-7. DOI
- Spetik M, Tekielska DA, Berraf-Tebbal A, Pecenka J, Stuskova K, Mahamedi AE, Eichmeier A. Diversity of Botryosphaeriaceae Species Associated with Grapevine Trunk Diseases in the Czech Republic. Diversity. 2023;5(7):800. DOI
- Moral J, Agustı-Brisach C, Perez-Rodrıguez M, Xavıer C, Raya-Ortega MC, Rhouma A, Trapero A. Identification of fungal species associated with branch dieback of olive and resistance of table cultivars to Neofusicoccum mediterraneum and Botryosphaeria dothidea. Plant Dis. 2017;101:306-16. DOI
- Moral J, Ahimera N, Felts DG, Morgan DP, Michailides TJ. Effects of wound size, amount of sap, and number of blighted nuts on infection of pistachio organs by Neofusicoccum mediterraneum. Plant Dis. 2017;101:2027-33. DOI
- Ahimera N, Driever GF, Michailides TJ. Relationships among propagule numbers of Botryosphaeria dothidea, latent infections, and severity of panicle and shoot blight in pistachio orchards. Plant Dis. 2003;87:846-53. DOI
- Ahimera N, Gisler S, Morgan DP, Michailides TJ. Effects of single-drop impactions and natural and simulated rains on the dispersal of Botryosphaeria Dothidea conidia. Phytopathology. 2004;94:1189-97. DOI
- Slippers B, Smit WA, Crous PW, Coutinho TA, Wingfield BD, Wingfield MJ. Taxonomy, phylogeny and identification of Botryosphaeriaceae associated with pome and stone fruit trees in South Africa and other regions of the world. Plant Pathol. 2007;56:128-39. DOI
- Mohammadi H, Sarcheshmehpour M, Mafi E. First record of Botryosphaeria dothidea associated with pistachio (Pistacia vera L.) panicle blight in Iran. J. Crop Prot. 2015;4:39-42. DOI
- Mohammadi H, Sarcheshmehpour M, Mafi E. Fungal trunk pathogens associated with wood decay of pistachio trees in Iran. Span. J. Agric. Res. 2015;13. DOI
- Chen SF, Morgan DP, Michailides TJ. Botryosphaeriaceae and Diaporthaceae associated with panicle and shoot blight of pistachio in California, USA. Fungal Divers. 2014;67:157-79. DOI
- Kovač M, Diminić D, Orlović S, Zlatković M. Botryosphaeria Dothidea and Neofusicoccum Yunnanense Causing Canker and Die-Back of Sequoiadendron Giganteum in Croatia. Forests. 2021;12(6):695. DOI
- Moral J, Muñoz-Dıez C, Gonzalez N, Trapero A, Michailides TJ. Characterization and pathogenicity of Botryosphaeriaceae species collected from olive and other hosts in Spain and California. Phytopathology. 2010;100:1340-51. DOI
- Michailides TJ, Morgan DP, Felts D, Hasey J, Puckett RD, Luo Y, Nouri MT, Luna M, Anderson K, Buchener R, Fichtner E, Coates W. Managing Botryosphaeria/Phomopsis canker and blight and anthracnose blight of walnut in California. In: Walnut Research Reports. California Walnut Board, Folsom, CA. 2014:299-317.
- Dıez CM, Moral J, Cabello D, Morello P, Rallo L, Barranco D. Cultivar and tree density as key factors in the long-term performance of super high-density olive orchards. Front. Plant Sci. 2016;7:54. DOI
- Moral J, Jurado-Bello J, Sanchez MI, Oliveira R, Trapero A. Effect of temperature, wetness duration, and planting density on olive anthracnose caused by Colletotrichum spp. Phytopathology. 2012;102:974-81. DOI
- Urbez-Torres JR, Peduto F, Vossen PM, Krueger WH, Gubler WD. Olive twig and branch dieback: etiology, incidence, and distribution in California. Plant Dis. 2013;97:231-44. DOI
- Urbez-Torres JR, Bruez E, Hurtado J, Gubler WD. Effect of temperature on conidial germination of Botryosphaeriaceae species infecting grapevines. Plant Dis. 2010;94:1476-84. DOI
- Agustı-Brisach C, Moral J, Felts D, Trapero A, Michailides T. Interaction between Diaporthe rhusicola and Neofusicoccum mediterraneum causing branch dieback and fruit blight of English walnut in California, and effect of pruning wounds to the infection. Plant Dis. 2019;103:1196-205. DOI
- Michailides TJ, Chen SF, Morgan D, Felts D, Nouri MT, Puckett R, Luna M, Hasey J, Anderson K, Coates W, Fichtner E, Buchner R, Bentley W. Managing Botryosphaeria/Phomopsis cankers and anthracnose blight of walnut in California. In: Proceedings of Walnut Board. Walnut Board of California, Folsom, CA. 2013:188-97.
- Michailides TJ, Morgan DP. Association of Botryosphaeriapanicle and shoot blight of pistachio with injuries of fruit caused by Hemiptera insects and birds. Plant Dis. 2016;100:1405-13. DOI
- Moral J, Morgan D, Michailides TJ. Management of Botryosphaeria canker and blight diseases of temperate zone nut crops. Crop Prot. 2019;8:104927. DOI
- Marsberg A, Kemler M, Jami F, Nagel JH, Postma-Smidt A, Naidoo S, Wingfield MJ, Crous PW, Spatafora JW, Hesse CN, Robbertse B, Slippers B. Botryosphaeria dothidea: a latent pathogen of global importance to woody plant health. Mol. Plant Pathol. 2017;18:477-88. DOI
- Piskur B, Pavlic D, Slippers B, Ogris N, Maresi G, Wingfield MJ, Jurc D. Diversity and pathogenicity of Botryosphaeriaceae on declining Ostrya carpinifolia in Slovenia and Italy following extreme weather conditions. Eur. J. Forest Res. 2011;130:235-49. DOI
- Stanosz GR, Blodgett JT, Smith DR, Kruger EL. Water stress and Sphaeropsis sapinea as latent pathogen of red pine seedlings. New Phytol. 2001;149:531-8. DOI
- Ma Z, Morgan DP, Michailides TJ. Effects of water stress on Botryosphaeria blight of pistachio caused by Botryosphaeria dothidea. Plant Dis. 2001;85:745-9. DOI
- Kaliterna J, Milicevic T, Ivic D, Bencic D, Mesic A. First report of Diplodia seriata as causal agent of olive dieback in Croatia. Plant Dis. 2013;97:231-44. DOI
- Allen RJ, Luptowitz R. El Niño-like teleconnection increases California precipitation in response to warming. Nat. Commun. 2017;8:16055. DOI
- Moral J, Lovera M, Benitez MJ, Arquero O, Trapero A. First report of Botryosphaeria obtusa causing fruit rot of quince (Cydonia oblonga) in Spain. Plant Pathol. 2007;56:351. DOI
- Brooks FE, Ferrin DM. Branch dieback of southern California chaparral vegetation caused by Botryosphaeria dothidea. Phytopathology. 1994;84:78-83. DOI
- English H. Relationship of Botryosphaeria dothidea and Hendersonula toruloidea to a canker disease of almond. Phytopathology. 1975;65:114-22. DOI
- Moral J, Perez-Rodrıguez M, Michailides TJ, Trapero A. First report of the teleomorph of Neofusicoccum mediterraneum, a pathogen of olive. In Phytopathology. 2015;105:S97.
- Michailides TJ, Morgan DP, Interbitzin P, Lampinen B, Reyes H, Puckett R, Windh J, Connell J, Duncan D, Holtz B, Adaskaveg J, Edstrom J, Krueger B, Viveros B, Verdegaal P, Browne G, McCoy D, Puget B, Post J. Etiology, Epidemiology, and Management of Lower Limb Dieback and Band Canker of Almonds. In: Production Research Report. California Pistachio Commission, Fresno, CA. 2009.
- Batista E, Lopes A, Alves A. Botryosphaeriaceae species on forest trees in Portugal: Diversity, distribution and pathogenicity. Eur. J. Plant Pathol. 2020;158:693–720. DOI
- Mahamedi AE, Phillips AJL, Lopes A, Djellid Y, Arkam M, Eichmeier A, Zitouni A, Alves A, Berraf-Tebbal A. Diversity, distribution and host association of Botryosphaeriaceae species causing oak decline across different forest ecosystems in Algeria. Eur. J. Plant. Pathol. 2020;158:745–65. DOI
- Belair M, Grau AL, Chong J, Tian X, Luo J, Guan X, Pensec F. Pathogenicity Factors of Botryosphaeriaceae Associated with Grapevine Trunk Diseases: New Developments on Their Action on Grapevine Defense Responses. Pathogens. 2022;11(8):951. DOI
- Nazar Pour F, Ferreira V, Felix C, Serodio J, Alves A, Duarte AS, Esteves AC. Effect of temperature on the phytotoxicity and cytotoxicity of Botryosphaeriaceae fungi. Fungal Biol. 2020;124:571–8. DOI
- Batista E, Lopes A, Alves A. What Do We Know about Botryosphaeriaceae? An Overview of a Worldwide Cured Dataset. Forests. 2021;12(3):313. DOI
- Zadoks JC, Schein RD. Epidemiology and plant disease management. Oxford UniversityPress, New York. 1979.
- Garcia JF, Lawrence DP, Morales-Cruz A, Travadon R, Minio A, Hernandez-Martinez R, Rolshausen PE, Baumgartner K, Cantu D. Phylogenomics of Plant-Associated Botryosphaeriaceae Species. Front. Microbiol. 2021;12:652802. DOI
- Leal L, Trotel-Aziz P, Gramaje D, Armengol J, Fontaine F. Exploring factors conditioning the expression of Botryosphaeria dieback in grapevine for integrated management of the disease. Phytopathology. 2024;114:21–34. DOI
- Bezerra JDP, Crous PW, Aiello D, Gullino ML, Polizzi G, Guarnaccia V, Genetic Diversity and Pathogenicity of Botryosphaeriaceae Species Associated with Symptomatic Citrus Plants in Europe. Plants. 2021;10(3):492. DOI
- Antony S, Billones-Baaijens R, Steel CC, Pathogenicity and progression of Botryosphaeriaceae associated with dieback in walnut orchards in Australia. Eur. J. Plant Pathol. 2024;168:723–42. DOI
- Moret F, Jacquens L, Larignon P, Clément G, Coppin C, Noirot E, Courty P-E, Fontaine F, Adrian M, Trouvelot S. Physiological and developmental disturbances caused by Botryosphaeria dieback in the annual stems of grapevine. Front. Plant Sci. 2024;15:1394821. DOI
- Lampinen B, Adaskaveg J, Browne G, Connell J, Duncan R, Michailides TJ, Metcalf S. Lower limb dieback in almond. in: Proceedings of Almond Board. Almond Board of California, Modesto, CA. 2009:178-85
- Garbelotto M. Drought heightens severity of diseases caused by Botryosphaeria dothidea and Cryptostroma corticale and needs to be factored in to properly assess pathogenicity or fulfill Koch’s postulates. J. Plant Pathol. 2024. DOI
- Liang D, Jiang Y, Zhang Y, Mao C, Ma T, Zhang C. The Comparative Genomics of Botryosphaeriaceae Suggests Gene Families of Botryosphaeria dothidea Related to Pathogenicity on Chinese Hickory Tree. J. Fungi. 2024;10(4):299. DOI
- Moral J, Eldesouki-Arafat I, Lopez-Escudero F, Vargas-Osuna E, Trapero A, Aldebis H, Olive escudete, caused by Botryosphaeria dothidea, as result of the interaction fly-mosquito-fungus. In Phytopathology. 2016;106:S135.
- Latinovic J, Mazzaglia A, Latinovic N, Ivanovic M, Gleason ML. Resistance of olive cultivars to Botryosphaeria dothidea, causal agent of olive fruit rot in Montenegro. Crop Prot. 2013;48:35-40. DOI
- Aldebis HK, Santos-Rufo A, Eldesouki-Arafat I, Vargas-Osuna E, Moral J, Trapero A, López-Escudero FJ. Olive Escudete (Dalmatian Disease) Caused by Botryosphaeria dothidea as a Result of Fly–Midge–Fungus Interaction. Horticulturae. 2024;10(4):321. DOI
- Wang X, Zhang W, Peng J. Lifestyle changes in Botryosphaeriaceae as evidenced by ancestral genome expansion and horizontal gene transfer. Fungal Divers. 2024;125:221-4. DOI
- Chen WQ, Morgan DP, Felts D, Michailides TJ. Antagonism of Paenibacillus lentimorbus to Botryosphaeria dothidea and biological control of panicle and shoot blight of pistachio. Plant Dis. 2003;87:359-65. DOI
- Chen SF, Fichtner E, Morgan DP, Michailides TJ. First report of Lasiodiplodia citricola and Neoscytalidium dimidiatum causing death of graft union of English walnut in California. Plant Dis. 2013;97:993. DOI
- Chen SF, Morgan D, Beede RH, Michailides TJ. First report of Lasiodiplodia theobromae associated with stem canker of almond in California. Plant Dis. 2013;97:994. DOI
- Adaskaveg JE, Gubler D, Michailides T. Fungicides, bactericides, and biologicals for deciduous tree fruit, nut, strawberry, and vine crops. University of California Agriculture and Natural Resources. 2017.
Cite this article:
Rhouma, A., Hajji-Hedfi, L., Khaire, P. B. Comprehensive analysis of Botryosphaeriaceae-induced panicle and shoot blight and its management strategies. DYSONA – Applied Science, 2025;6(1): 40-50. doi: 10.30493/das.2024.472342

