Lamiaa A. Mutlag1, Raghad S. Mouhamad1*, Saadi M. Al-Ghrairi1
1, Agricultural Research Directorate, Ministry of Science and Technology, Baghdad, Iraq
E-mail:
raghad1974@yahoo.com
Received: 08/03/2020
Acceptance: 28/05/2020
Available Online: 29/05/2020
Published: 01/07/2020

Manuscript link
http://dx.doi.org/10.30493/DAS.2020.222762
Abstract
Plant nutrition is one of the determinate factors in fruit production cycles. Foliar fertilizer application is considered a complimentary nutrition method which proofed to be highly efficient under certain conditions. In this research, a foliar application of balanced foliar fertilizer N.P.K (20:20:20) +TE with different concentrations (0, 5, 10, and 15 g/l) was conducted on Coscia pear seedlings. Plant vegetative growth traits in addition to certain biochemical characteristics (Roots and leaves dry weight percentages, chlorophyll content, carbohydrates content, and minerals content) were measured. The results showed that plant height, stem diameter, branches length, leaf area, main roots number, root length, root dry weight, leaves dry weight, chlorophyll, and minerals content were significantly increased by increasing foliar spray concentration from 5 to15 g/l which confirms the importance of foliar spray as an enhancer of plant physiological operations. However, the highest carbohydrate content and carbohydrate/nitrogen (C/N) ratio were observed under A10 (10 g/l) treatment which might refer to the importance of this level of foliar fertilization, specifically in this phase, to accelerate the transition from juvenility to production phase.
Keywords: Foliar application, Nutrition, Pyrus communis
Introduction
Pear (Pyrus communis L.) belongs to the family Rosaceae and was originated in the areas surrounding the Caspian Sea and the northern foothills of the Himalayas, from which it spread to southern Europe [1].
Plant nutrition is one of the most important factors affecting the growth and productivity of fruit trees in general. In order for the plant to grow, develop and complete its life cycle ideally, there is a need for a permanent supply of nutrients that are the driving forces of all the vital activities carried out by the plant. Therefore, nutrient deficiencies adversely affect the growth and development of the plant [2-4].
Nitrogen, phosphorus, and potassium are among the most important nutrients. Nitrogen plays the vital role in the synthesis of amino acids, nucleic acids, chlorophyll, and many plant hormones [5-9]. The importance of phosphorus lies in the formation of many organic compounds necessary such as energy-rich compounds such, and plays a key role in photosynthesis, respiration, in addition to carbohydrate and fatty acids synthesis [10][11].
Some of the nutrients present in or added to the soil are exposed to sedimentation, washing, or volatilization, especially in soils with a basic reaction or a high content of lime [12]. Therefore, the addition of fertilizers of macro and microelements to soils in central and southern Iraq, which is characterized by its high content of lime with a basic reaction as well as its hot and dry climate in summer, is often accompanied by a large loss of these nutrients.
Since the leaf is the center of photosynthesis and other vital processes, the lack of nutrients promptly appears on the leaves; therefore, the fastest way to address this deficiency is by adding nutrients directly through foliar fertilizing [13][14] which is carried out by spraying the vegetative system with completely soluble forms of nutrients and can meet up to 85% of plant’s nutrients requirements [15]. Previously, Bentchikou [16] and Kessel [17] indicated that the primary goal of foliar fertilization is to facilitate absorption and utilization of the used nutrients and to remove the visible deficiency symptoms on the leaves due to the lack in one or more of the nutrients in addition to improving the growth and yield [18][19]. This method is considered economically efficient as it reduces the need for large quantities of nutrients, especially macro elements, in comparison to other methods [20], ensures the rapid positive response of the vegetative parts, and enables the mixing of fertilizers with pesticides and growth regulators which reduces energy and time consumption. Therefore, this study aimed to investigate the effects of different levels of foliar fertilization on the early vegetative characteristics in Coscia pear seedlings
Materials and Methods
The experiment was carried out at 2015 season in the Plant Breeding and Improvement Research Center, Directorate of Agricultural Research, Ministry of Science and Technology,Al–Tuwaitha station, Baghdad, Iraq, to study the response of Coscia pear seedlings to the foliar fertilization. The seedlings were grafted on Pyrus calleryana rootstocks at the age of one year and planted in 10 kg black plastic bags, filled with soil and peat (1:1).
Foliar fertilizer application
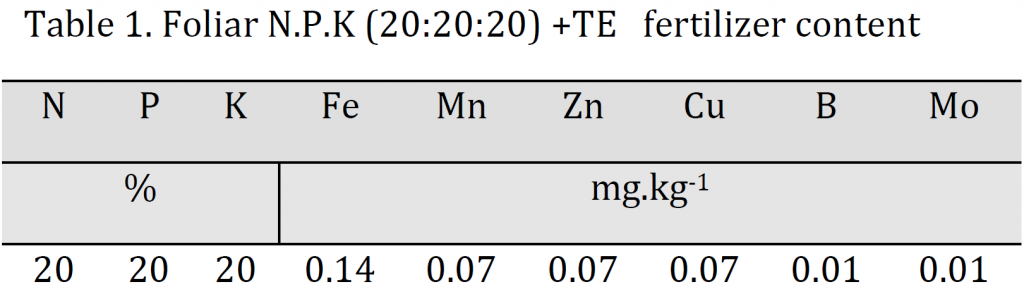
A balanced foliar fertilizer (20-20-20) nutrient solution (Scotts–Bulgaria) with the contents shown in (Table 1) was used in this experiment. Foliar spraying was carried out every 21 days from the first week of April 2015 to the beginning of October 2015. Each application was carried out in the early morning one day after irrigation as the stomata are open and the leaf soluble contents concentrations are low which promotes the process of absorption. The solution was sprayed onto the vegetative system using a 10-liter manual sprinkler until full wetness with the addition of Tween-20 to reduce the surface tension of the water molecules. Three levels of foliar spray concentrations were used 5 g/l (A5), 10 g/l (A10), and 15 g/l (A15) in addition to a control treatment (A0).
Vegetative characteristics measurements
Plant height was measured using a metric tape measure, from grafting area to the top of the plant, before and after the experiment. The stem diameter was measured before and after the experiment at a height of 5 cm above the grafting area. Branches length for each experimental unit was measured before and after the experiment. Leaf area was measured as an average of 10 leaves from different directions from each experimental unit. The total leaf area of the plant was calculated by multiplying the average area of one leaf by the total number of leaves for the plant. The main roots were counted after the experiment. For this purpose, the seedlings were extracted from the soil and placed in water for cleaning while ensuring that the root system is preserved. Root length was measured using the metric tape. Samples of leaves and roots were placed in paper bags and left in a ventilated room for 24 hours, then dried in an electric oven at a temperature of 70 ○C until the weight became constant, and then the dry weight of both sample types was calculated
Dry weight % = [Dry weight / (Fresh weight – Dry weight)] X 100 as in [21].
Biochemical measurements
The leaves chlorophyll content was measured using 502-SPAD Chlorophyll meter (Minolta-Japan) by taking the reads of 10 leaves per experimental unit and then calculating the average according to [22].
For other biochemical assays, samples of 5 leaves from each experimental unit were dried in an electric oven at a temperature of 65 ○C for 72 hours until the weight became constant and then dried leaves were powdered.
Carbohydrates content in the leaves was measured according to [22] by adding 12 ml of (1N) perchloric acid to 0.2 g of dry leaves powder. The samples were then incubated in a water bath at 60 ○C for 60 minutes. After that, the samples were centrifuged for 15 minutes at 3000 rpm, and then, the clear supernatant was collected in a volumetric flask. The previous steps were repeated thrice for each sample, and then the flask volume was made up to 100 ml by adding distilled water. 1 ml of the previous extract was taken and 1 ml of 5% phenol alcohol with 5 ml of concentrated sulfuric acid was added. The light absorption of the formed solutions was then measured at 490 nm wavelength with UV/VIS spectrometer. Carbohydrates content was calculated based on the standard absorbance equation and the original sample weight.
To evaluate mineral content, 0.2 g of the powdered sample was digested using the method described in [15]. Nitrogen was estimated using micro Kjeldahl apparatus according to the method described in [14]. C/N ratio was calculated by dividing the carbohydrate content on nitrogen content. Potassium content was estimated by flame photometer according to the method proposed [22]. Total phosphorus was measured according to ammonium molybdate test proposed by [14] by measuring light absorption at a wavelength of 880 nm. Iron, zinc, calcium, and magnesium contents were estimated by the atomic absorption spectrophotometer according to the method mentioned in [22].
Experimental design and statistical analysis
The experiment was carried out in a Randomized Complete Block Design (RCBD). Each measurement was replicated three times. The averages were compared using Fisher’s least significant difference (LSD) at a probability level of P<0.05.
Results and Discussion
Vegetative growth characteristics
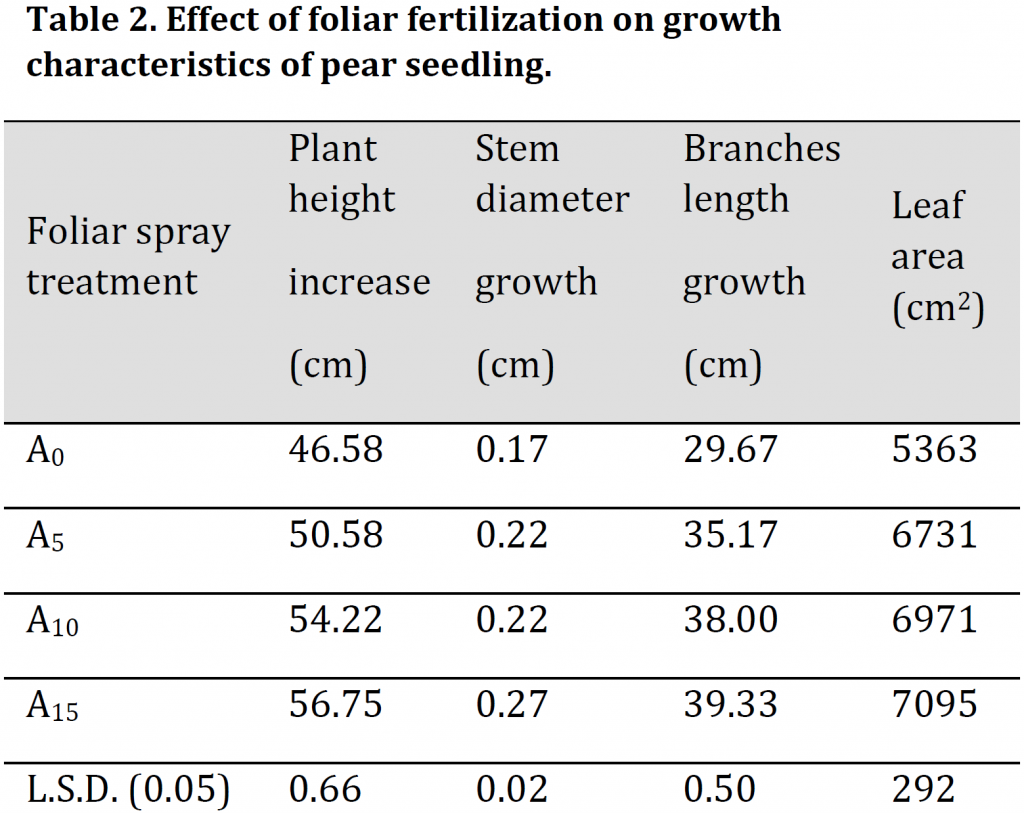
The results indicated that A15 treatment (15 g/l) induced the most significant increase in plant height, stem diameter, branches length, and leaf area with values of 56.75 cm, 0.27 cm, 0.4 mm, 39.33 cm, and 7095 cm2 for each trait respectively; while the lowest values were attributed to A0 with 46.58 cm, 0.17 cm, 0.32 mm, 29.67 cm, 5363 cm2 for the previously mentioned traits respectively (Table 2). Overall, a significant increase in all vegetative traits was observed by each increase in foliar spray concentration; however, leaf area and stem did not undergo an increase between A5 and A10 treatments (Table 2). The observed increase in the evaluated traits is attributed to the role of nutrient solution in satisfying part of the plant’s need for mineral nutrients necessary for photosynthesis, respiration, and various metabolic processes; especially nitrogen role in stimulating auxin synthesis, which encourages cell division and elongation. These results are consistent with [3][5][22].
Root characteristics
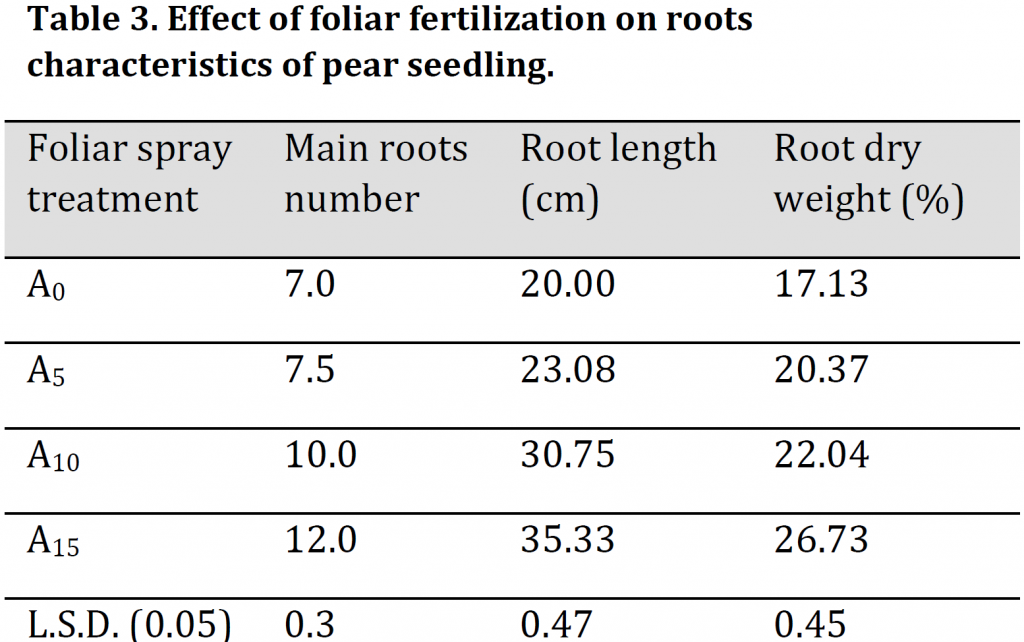
It was noticed that foliar fertilizer spray had significantly increased main roots number, length, and dry weight percentage. The highest values of the previously mentioned characteristics were observed in A15 with 12, 35.33 cm, and 26.73 % respectively while the lowest values were recorded under A0 treatment with 7, 20 cm, and 17.13 % respectively (Table 3). These results of significant increase in the number, length, and dry weight of the roots under foliar fertilizer application are consistent with [2].
Biochemical characteristics and mineral content
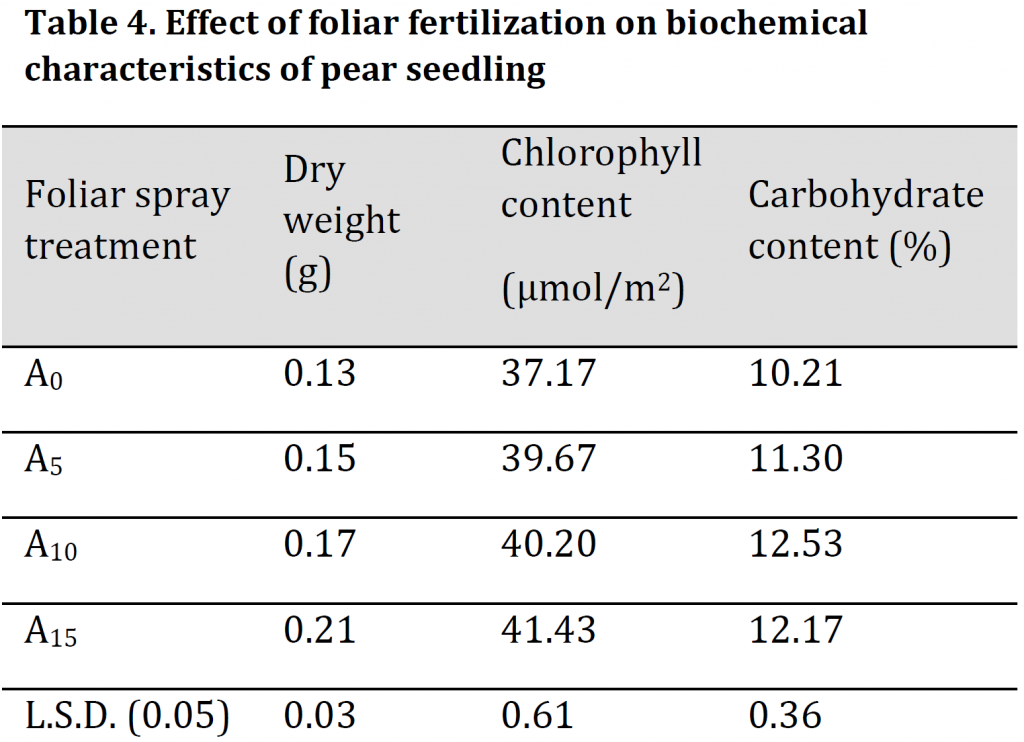
Leaves’ dry weight and chlorophyll contents were significantly increased by increasing foliar application with A15 treated seedlings having the highest values of 0.21g and 41.43 µmol/m2 respectively and A0 treated seedlings with the lowest values 0.13 g and 37.17 µmol/m2 (Table 4). Similarly, carbohydrate content in leaves was also significantly increased under foliar spray treatment. Although A15 treated seedlings had significantly higher carbohydrate content in comparison to both A0 and A5 treatments, the highest carbohydrate content was found in A10 treatment (Table 4). The reason may be due to the formation of some enzymatic accompaniments that contribute to stimulating growth (NADPH, NADP, FAD). Additionally, potassium by controlling the opening and closing of stomata increases the efficiency of the leaf in photosynthesis and carbohydrate production. These results are similar to those obtained by [23][24]
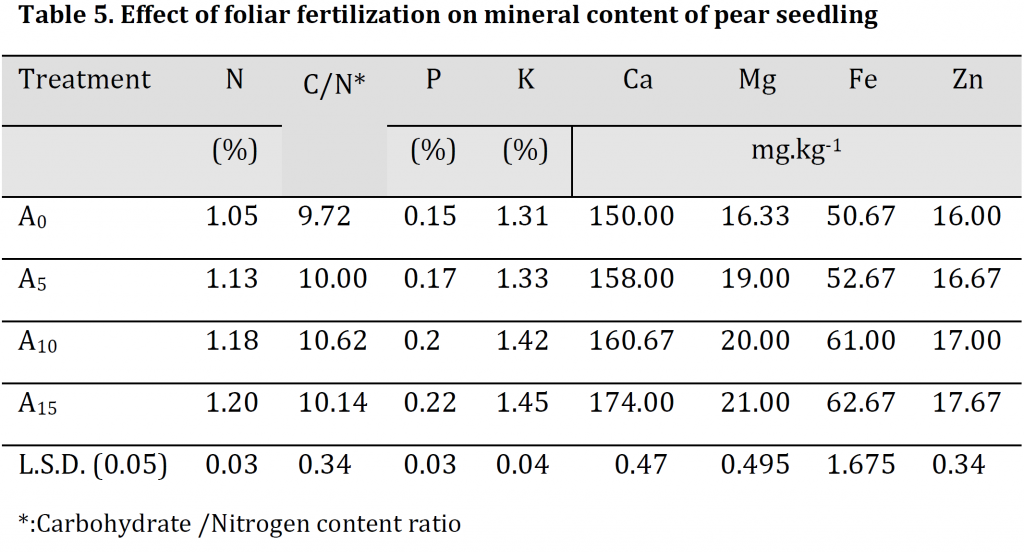
Mineral content analysis results illustrated that the increase in foliar fertilizer concentration resulted in significant increases in leaves minerals contents (Table 5). The highest nitrogen, phosphorus, potassium, calcium, magnesium, iron and zinc were observed in A15 treatment with values of 1.2 %, 0.22 %, 1.45 %, 174 mg.kg-1, 21 mg.kg-1, 62.67 mg.kg-1, and 17.67 mg.kg-1 respectively; while the lowest values were recorded in A0 treatment with 1.05 %, 0.15 %, 1.31 %, 150 mg.kg-1, 16.33 mg.kg-1, 50.67 mg.kg-1, and 16 mg.kg-1 respectively. A significant increase in the carbohydrate/nitrogen (C/N) ratio was observed by increasing the dose of foliar spray. However, due to the significantly higher carbohydrate and lower nitrogen contents in A10 treatment in comparison to A15 treatment; C/N ratio was significantly higher in the former treatment in comparison to the later (Table 5) which might refer to the importance of this treatment level in accelerating transition period from juvenility to production phase [25]. Foliar fertilizer was previously reported to increase the plant’s nutrient content as the macro and micronutrients that are absorbed directly through the leaves stimulate the root system leading to an absorption increase and an assimilation enhancement, which positively influences the absorption of potassium and phosphorous, and this explains the increase in mineral content in the leaves [24]. Additionally, the enhancements in the nutritional condition seem to have had a significant positive effect on photosynthesis that reflected as an increase in carbohydrate content (Table 5) which is consistent with [19][26].
References
| 1 | Zohary D. Wild apples and wild pears. Bocconea. 1997;7:409-16. |
| 2 | Al-Ajil SA. Effect of salinity, organic waste and nutrition Foliagein tomato plants in the desert region of Najaf. PhD thesis.College Agriculture.Baghdad University. 1998. |
| 3 | Mouhamad R, Atiyah A, Iqbal M. Behavior of Potassium in Soil: A mini review. Chemistry International. 2016;2(1):58-69. |
| 4 | Mahdi HH, Mouhamad RS. Behavior of Phosphorus in the Calcareous Soil. Advances in Agricultural Technology & Plant Sciences 2018;1(4): 180018. |
| 5 | Goffart JP, Olivier M, Frankinet M. Potato crop nitrogen status assessment to improve N fertilization management and efficiency: past–present–future. Potato Res. 2008;51(3-4):355-83. DOI |
| 6 | Havlin, J. L.; J. D. Beaton; S. L. Tisdale and W. L. Nelson. Soil Fertilizers. 7th ed. Upper Saddle River, New Jersey. 2005. |
| 7 | Bal, J. S. Fruit Growing. 3rd edt. Kalyani Publishers, New Delhi -110002. 2005. |
| 8 | Mouhamad RS, Atiyah AH, Hasan AF, Elkaaby EA. Changes in Macro Elements Content in Plant Tissues Subjected to Clinostat. J Rice Res. 2019;7(209):2. DOI |
| 9 | Mouhamad RS, Rasheed AG, AL-Gburi HF, Nazir A, Iqbal M, Razaq I. Predicting Soil Organic Carbon Turnover in Soils at the Middle Region of Iraqi Using Infrared Spectra. Jacobs Journal of Plant Biology. 2019;4(1):009. |
| 10 | Barker AV, Pilbeam DJ. Handbook of plant nutrition. CRC press. 2015. |
| 11 | Mouhamad RS, Shallal HS, Al-Daoude A. Microgravity Effects on the Growth, Cell Cytology Properties and DNA Alterations of Two Iraqi Local Plants. J Rice Res. 2019;7(207):2. DOI |
| 12 | Mengel K. Alternative or complementary role of foliar supply in mineral nutrition. In International Symposium on Foliar Nutrition of Perennial Fruit Plants 594. 2001:33-47. DOI |
| 13 | Hamad MS, Faruq F. The effect of foliar fertilization on mineral content and proportion of nodes of local orange trees (Citrus sinensisosbeck). Journal of Agricultural Sciences. 2000; 31(2). |
| 14 | Ali HZ, Mohammed RS, Aboud HM. Efficiency of organic matter levels and bio fungus Trichoderma harzianum on cucumber plant. IOSR Journal of Agriculture and Veterinary Science. 2015;8(6):28-34. DOI |
| 15 | Abdul Karim S. Physiological nutrients. Dar Al-Kutub for Printing and Publishing, Salahuddin University, Ministry of Higher Education and Scientific Research, Iraq. 1988. |
| 16 | Bentchikou ME. Influence sur quelques aspects de la physiologie de la vigne d’un apport par voie foliaire de substances minérales et organiques. Doctoral dissertation, Bordeaux 2. 1990. |
| 17 | Kessel C. Strawberry Diagnostic Workshops, Nutrition. Ministry of Agriculture. Food and Rural Affaies. 2006:1-7. |
| 18 | Mouhamad RS, Jaafar ZM, El–Kaaby EA, Iqbal M, Arif N. Evaluation of Agronomic Traits and Inorganic Nutritional Composition of Rice Seed from IRSSTN Genotypes in Iraq. J Rice Res. 2018;6(189):2. DOI |
| 19 | Fallahi E, Eichert T. Principles and practices of foliar nutrients with an emphasis on nitrogen and calcium sprays in apple. HortTechnology. 2013;23(5):542-7. DOI |
| 20 | Zargar M, Tumanyan A, Ivanenko E, Dronik A, Tyutyuma N, Pakina E. Impact of foliar fertilization on apple and pear trees in reconciling productivity and alleviation of environmental concerns under arid conditions. Commun. Integr. Biol. 2019;12(1):1-9. DOI |
| 21 | Shaheen R, Al-Radhiman KN. Practical exercises in plant nutrition. Scientific publishing and printing – King Saud University. 2001. |
| 22 | Hassan HS, Sarrwy SM, Mostafa EA. Effect of foliar spraying with liquid organic fertilizer, some micronutrients, and gibberellins on leaf mineral content, fruit set, yield, and fruit quality of “Hollywood” plum trees. Agriculture and Biology Journal of North America. 2010;1(4):638-43. |
| 23 | Al-Isawi SA, Helmi IM, Al-Rawi WA. Effect of foliar spraying of TOTALGRO and Gibberellic Acid on growth and yield of Apple Malus domestica cvs. Sharabi and Anna 1. Leaf area and NP K contents. Anbar Journal of Agricultural Sciences. 2011;9(2):197-208. |
| 24 | Alexander A, Hunsche M. Influence of formulation on the cuticular penetration and on spray deposit properties of manganese and zinc foliar fertilizers. Agronomy. 2016;6(3):39. DOI |
| 25 | Higazy MK. Shortening the juvenile phase for flowering. Doctoral dissertation, Veenman. 1962. available from: Link |
| 26 | Fawzi M, Shahin F, Elham A, Kandil E. Effect of organic and bio-fertilizers and magnesium sulphate on growth yield, chemical composition and fruit quality of” Le-Conte” Pear trees. Nature and Science. 2010;8(12):273-80. |
Cite this article:
Mutlag, L., Mouhamad, R., Al-Ghrairi, S. Effect of foliar fertilization in Coscia Pear seedlings on vegetative growth and biochemical characteristics. DYSONA – Applied Science, 2020;1(2): 51-56. doi: 10.30493/das.2020.222762
