Vivek Darapaneni 1*; Anusha Jaldani 2
1, Department of Virology and Computational Biochemistry, Anvek Institute of Biomolecular Research, Visakhapatnam, India
2, Department of Computational Pharmacology, Anvek Institute of Biomolecular Research, Visakhapatnam, India
E-mail:
vivek110385@gmail.com
Received: 21/02/2022
Acceptance: 02/03/2022
Available Online: 03/03/2022
Published: 01/04/2022

Manuscript link
http://dx.doi.org/10.30493/DLS.2022.330732
Abstract
Influenza A virus (IAV) is an etiological agent which causes respiratory tract infection in humans and other animals. In the past, IAV was responsible for seasonal epidemics and periodical pandemics. IAV non-structural protein 1 (NS1) is a multifaceted protein that plays a pivotal role in the infectious life cycle of IAV. For the multifunctional role in establishing the IAV infection, NS1 has to translocate between nucleus and cytoplasm with the help of human importin α protein. In this study, we tested ivermectin for its ability to bind with IAV NS1 protein. The results showed that ivermectin binds with IAV NS1 with a high AutoDock Vina score of -7.3 kcal/mol. The identified novel ivermectin binding site has been found in the area overlapping NLS1 of NS1 protein. Targeting the identified site on the NS1 protein with ivermectin could inhibit NS1- importin α protein interaction which can have a deleterious effect on the infectious life cycle of IAV. Moreover, the identified binding site is universally conserved and resistant to mutations that could arise in the future. This finding renders ivermectin a promising universal anti-IAV drug molecule.
Keywords: Influenza virus, Non-structural protein 1, importin α protein, Ivermectin, Molecular docking
Introduction
Influenza viruses are enveloped viruses belonging to the Orthomyxoviridae family with a negative-sense, single-stranded, segmented RNA genome [1]. Influenza viruses are classified into four genera: influenza A, B, C, and D viruses [2]. Characterized with eight viral genomic segments, Influenza A virus (IAV) is known to cause respiratory infection in a wide range of hosts, including humans. IAV is known to cause seasonal epidemics and occasional pandemics in the human population and is a cause of concern with respect to general human health and well-being [3].
Non-structural protein 1 (NS1) is a multifaceted protein with a molecular weight of approximately 26 kDa and a sequence length of 215 to 237 residues encoded by the eighth segment of the IAV genome [4]. Ns1 protein consists of two domains, namely N-terminal domain (RNA binding domain; residues 1-73) and C-terminal domain (effector domain; residues 85-207) connected by a linker region (residues 74-84), while the final portion of residues (208-230/237) represent a disordered tail region [4]. NS1 protein contains two nuclear localization signal (NLS) namely NLS1 (residues 34-38) and NLS2/nucleolar localization signal (NoLS) (residues 216-237) in addition to a nuclear export signal (NES; residues 134-161) [5, 6]. NS1 protein plays an important role in suppressing host immunity, shutting off the expression of host genes, apoptosis, viral replication, and determination of virulence [7].
Ivermectin, an antiparasitic drug widely used in veterinary medicine owing to its broad-spectrum, safety, and efficacy [8], has been shown to have antiviral action in a wide variety of viruses. Ivermectin is an inhibitor of importin α/β-mediated nuclear import [9] and was reported to inhibit the nuclear import of human immunodeficiency virus 1 (HIV-1) integrase [9], simian virus 40 large tumor antigen (SV40 TAg) [10], dengue virus non-structural protein 5 (DENV NS5) [9], and block nuclear location signal in parvovirus [11]. Furthermore, ivermectin was reported to impede infection of dengue virus 1-4 [12], west Nile virus [13], Venezuelan equine encephalitis virus [14], influenza virus [15], pseudorabies virus [16], and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [17].
Four potential binding sites of ivermectin were found previously on the matrix protein 1 binding domain of the nuclear export protein (NEP) through structural similarity between the IAV NEP C-terminal domain and the structures with ivermectin as heteroatoms in the protein data bank [18]. In this study, IAV NS1 protein was targeted for drug repurposing study. Ivermectin was tested for its ability to bind with IAV NS1 protein utilizing in silico molecular docking technique.
Methods
IAV NS1 and ivermectin structure retrieval
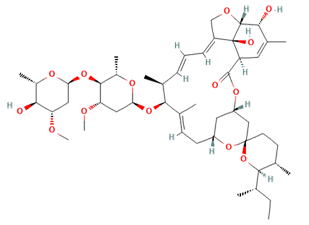
The protein structure of IAV NS1 was retrieved from the protein data bank with PDB identifier 6O01 (https://www.rcsb.org/structure/6O01) [19]. The obtained .pdb file consisted of one chain, namely chain A. Residues 4-199 of IAV NS1 makeup chain A. Ivermectin structure (.sdf file) was retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov/compound/6321424) with PubChem CID 6321424 [20] (Fig. 1).
Molecular docking
The IAV NEP was docked with ivermectin using CB-Dock docking server (http://cao.labshare.cn/cb-dock/) [21]. CB-Dock is a protein-ligand docking server performing blind docking involving four steps. First, putative binding sites are detected on the protein structure. Then, top binding sites are selected. The selection is based on the size of the binding sites. After that, the docking center is calculated, and the docking box size is adjusted. Finally, docking is carried out using AutoDock Vina, and after completion of docking, binding sites are ranked according to docking score [21].
Ivermectin binding site conservation analysis
IAV NS1 protein sequences were downloaded from National Centre for Biotechnology Information (NCBI) influenza virus resource [22]. Full-length sequences of NS1 from all hosts and subtypes were chosen, while duplicate protein sequences were removed. MAFFT version 7 was used with default parameters to align the obtained NS1 protein sequences [23].
Results and Discussion
Ivermectin binding site
A total of five binding sites for ivermectin were identified on NS1 with varying Vina scores. By default, the first conformation with the highest score is treated as the best binding position, and the corresponding site is the optimal binding site for ivermectin [21]. Ivermectin was found to bind IAV NS1 with a high AutoDock Vina score of -7.3 kcal/mol [cavity size: 194Å, center: -14*73*-46 (x*y*z) and size: 29*29*29 (x*y*z)] (Fig. 2). The ivermectin binding site residues identified on NS1 protein are Trp16, Lys20, Leu33, Leu36, Arg37, Asp39, Gln40, Leu43, Arg44 and Ile54.
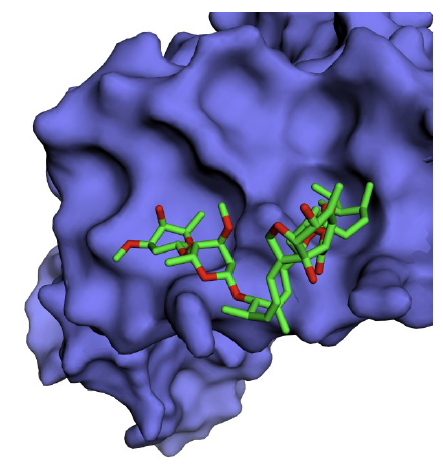
Binding site conservation
A total of 17549 unique IAV NS1 protein sequences were obtained. Multiple sequence alignment was carried out using MAFFT version 7 [23]. Amino acid variation analysis was carried out at positions corresponding to the predicted ivermectin binding site on NS1 (Table 1).
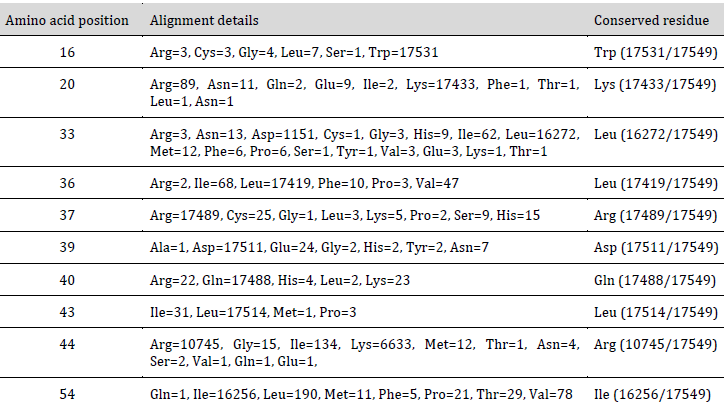
Among the residues lining the ivermectin binding site, Trp16, Lys20, Leu36, Arg37, Asp39, Gln40, and Leu43 residues were found to be conserved. Residues Leu33 and Ile54 were found to be intermediately conserved, while residue Arg44 was found to be highly variable across various IAV hosts. Therefore, from the predicted binding site residues, seven are conserved, two are intermediately conserved, and only one residue is highly variable. Universally, the predicted ivermectin binding site is lined by conserved residues. These conserved residues might have either functional or structural importance, which might render them resistant to change in the evolution of the NS1 protein. Therefore this binding site is predicted to be highly resistant to future mutations.
The predicted binding site of ivermectin spans the NLS1 region of NS1 was reported to interact with human importin α protein and translocate across the membrane to the nucleus [24]. Thus, targeting this site with ivermectin might interfere with the translocation of NS1 protein. Targeting the IAV NS1 with ivermectin would probably have a deleterious effect on the infectious life cycle of IAV. Moreover, the predicted ivermectin binding site is mutation resistant. This makes ivermectin a promising universal anti-IAV drug molecule.
Conclusion
This study revealed a novel ivermectin binding site in the area overlapping NLS1 of NS1 protein. The identified binding site is universally conserved and resistant to mutations that could arise in the future. Ivermectin was found to bind with IAV NS1 protein strongly. It is anticipated that blocking the NS1 – importin α protein interaction can have a considerable deleterious effect on IAV NS1 protein dimerization and its nuclear import. Thereby the function of NS1 protein in the infectious life cycle of IAV would be disrupted, affecting the survival of IAV in the infected cell.
References
| 1 | Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses. Fields Virology. Lippincott-Williams & Wilkins, Philadelphia. 2013:1186-1243. DOI |
| 2 | Hause BM, Collin EA, Liu R, Huang B, Sheng Z, Lu W, Wang D, Nelson EA, Li F. Characterization of a novel influenza virus in cattle and Swine: proposal for a new genus in the Orthomyxoviridae family. MBio. 2014;5(2):e00031-14. DOI |
| 3 | Harrington WN, Kackos CM, Webby RJ. The evolution and future of influenza pandemic preparedness. Exp. Mol. Med. 2021;53(5):737-49. DOI |
| 4 | Hale BG, Randall RE, Ortín J, Jackson D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 2008;89(10):2359-76. DOI |
| 5 | Li Y, Yamakita Y, Krug RM. Regulation of a nuclear export signal by an adjacent inhibitory sequence: the effector domain of the influenza virus NS1 protein. Proc. Natl. Acad. Sci. 1998;95(9):4864-9. DOI |
| 6 | Melén K, Kinnunen L, Fagerlund R, Ikonen N, Twu KY, Krug RM, Julkunen I. Nuclear and nucleolar targeting of influenza A virus NS1 protein: striking differences between different virus subtypes. J. Virol. 2007;81(11):5995-6006. DOI |
| 7 | Hao W, Wang L, Li S. Roles of the non-structural proteins of influenza A virus. Pathogens. 2020;9(10):812. DOI |
| 8 | González Canga A, Sahagún Prieto AM, Diez Liébana MJ, Fernández Martínez N, Sierra Vega M, García Vieitez JJ. The pharmacokinetics and interactions of ivermectin in humans—a mini-review. AAPS J. 2008;10(1):42-6. DOI |
| 9 | Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012;443(3):851-6. DOI |
| 10 | Wagstaff KM, Rawlinson SM, Hearps AC, Jans DA. An AlphaScreen®-based assay for high-throughput screening for specific inhibitors of nuclear import. J Biomol Screen. 2011;16(2):192-200. DOI |
| 11 | Nguyen KY, Sakuna K, Kinobe R, Owens L. Ivermectin blocks the nuclear location signal of parvoviruses in crayfish, Cherax quadricarinatus. Aquaculture. 2014;420:288-94. DOI |
| 12 | Tay MY, Fraser JE, Chan WK, Moreland NJ, Rathore AP, Wang C, Vasudevan SG, Jans DA. Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Res. 2013;99(3):301-6. DOI |
| 13 | Yang SN, Atkinson SC, Wang C, Lee A, Bogoyevitch MA, Borg NA, Jans DA. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020;177:104760. DOI |
| 14 | Lundberg L, Pinkham C, Baer A, Amaya M, Narayanan A, Wagstaff KM, Jans DA, Kehn-Hall K. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication. Antiviral Res. 2013;100(3):662-72. DOI |
| 15 | Götz V, Magar L, Dornfeld D, Giese S, Pohlmann A, Höper D, Kong BW, Jans DA, Beer M, Haller O, Schwemmle M. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci. Rep. 2016;6(1):1-5. DOI |
| 16 | Lv C, Liu W, Wang B, Dang R, Qiu L, Ren J, Yan C, Yang Z, Wang X. Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antiviral Res. 2018;159:55-62. DOI |
| 17 | Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. DOI |
| 18 | Darapaneni, V. Novel Ivermectin Drug Binding Sites in the C-terminal Domain of Influenza A Virus Nuclear Export Protein. Am. J. Curr. Virol. 2015;1:13-19. |
| 19 | Mitra S, Kumar D, Hu L, Sankaran B, Moosa MM, Rice AP, Ferreon JC, Ferreon AC, Prasad BV. Influenza A virus protein NS1 exhibits strain-independent conformational plasticity. J. Virol. 2019;93(21):e00917-19. DOI |
| 20 | Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, Han L, He J, He S, Shoemaker BA, Wang J. PubChem substance and compound databases. Nucleic Acids Res. 2016;44(D1):D1202-13. DOI |
| 21 | Liu Y, Grimm M, Dai WT, Hou MC, Xiao ZX, Cao Y. CB-Dock: a web server for cavity detection-guided protein–ligand blind docking. Acta Pharmacol. Sin. 2020;41(1):138-44. DOI |
| 22 | Bao Y, Bolotov P, Dernovoy D, Kiryutin B, Zaslavsky L, Tatusova T, Ostell J, Lipman D. The influenza virus resource at the National Center for Biotechnology Information. J. Virol. 2008;82(2):596-601. DOI |
| 23 | Rozewicki J, Li S, Amada KM, Standley DM, Katoh K. MAFFT-DASH: integrated protein sequence and structural alignment. Nucleic Acids Res. 2019;47(W1):W5-10. DOI |
| 24 | Marc D. Influenza virus non-structural protein NS1: interferon antagonism and beyond. J. Gen. Virol. 2014;95(12):2594-611. DOI |
Cite this article:
Darapaneni, V., Jaldani, A. In silico binding site detection of ivermectin with influenza A virus NS1 protein. DYSONA – Life Science, 2022;3(1): 36-40. doi: 10.30493/dls.2022.330732
