Mohsen Abbod 1*; Issam Alkhouri 2; Rasha Shahoud 2
1, Department of Plant Protection, Faculty of Agricultural Engineering, Al-Baath University, Homs, Syria
2, Department of Soil and Land Reclamation, Faculty of Agricultural Engineering, Al-Baath University, Homs, Syria
E-mail:
abbod.mohsen111@gmail.com
Received: 26/08/2024
Acceptance: 14/09/2024
Available Online: 15/09/2024
Published: 01/01/2025

Manuscript link
http://dx.doi.org/10.30493/DAS.2024.475340
Abstract
Maize (Zea mays L.) is a primary cereal crop in achieving food security worldwide. This plant is currently facing a multitude of threats from various plant pathogens, resulting in significant crop loss, particularly due to root rot diseases. This study aimed to evaluate the efficacy of the biocontrol agent Trichoderma harzianum at two concentrations, 5×10+5 (T1) and 5×10+6 (T2), with or without plant compost (Co), on the growth parameters of maize plants infested (P) with the pathogenic fungus Fusarium solani under greenhouse conditions. The dual culture assay revealed a substantial antagonistic effect against F. solani with 55% and 81% of mycelial growth inhibition after 4 and 7 days, respectively. The greenhouse experiment demonstrated that the treatment utilizing a combination of T. harzianum and plant compost resulted in shoot and root wet and dry weights comparable to those of healthy (uninfected) plants (C). Notably, the P+T2+Co treatment yielded a root wet weight of 28.37±0.34g and a dry weight of 19.76±0.78g, surpassing the readings in healthy plants (26±0.69g and 16.9±1.7g, respectively); however, this increase was not statistically significant in either case. The results unequivocally demonstrate the critical importance of utilizing T. harzianum as a biocontrol agent and plant-derived compost as an effective biofertilizer for maize plants subjected to the biotic stress of Fusarium solani infection. Consequently, further studies are necessary to evaluate the antagonistic activity of T. harzianum under field conditions.
Keywords: Trichoderma harzianum, Fusarium solani, Biocontrol agent, Compost, Dual culture, Antagonistic activity
Introduction
Maize (Zea mays L.) serves as an essential grain crop for ensuring food security. This crop suffers a substantial yield reduction due to the presence of multiple pests and pathogens, including the Fusarium genus. This fungal genus poses a significant threat, leading to diseases such as Fusarium ear rot, root rot, and stalk rot [1], and resulting in considerable losses in maize yield in major producing countries [1][2]. Fusarium diseases often result in root and crown rot, especially when roots are damaged [1-3]. Fusarium root rot, induced by F. solani, is a prevalent corn pathogen, particularly in humid regions, leading to wilting and significant yield reduction. Affected plants may exhibit stunted growth and desiccated leaves, while specific Fusarium isolates generate deleterious mycotoxins that adversely affect both animals and humans.
The excessive use of agrochemicals has led to detrimental impacts on the ecosystem and human health. Consequently, initiatives in sustainable agriculture have focused on identifying safer and ecologically viable control alternatives, particularly the use of biological control agents (BCAs) for soil-borne pathogens [4-6]. These beneficial microorganisms, including fungi and bacteria, inhibit the infection and emergence of pathogens within the host plant or soil [7-10]. There has been a significant rise in the use of fungal biological control agents to address plant pathogenic fungi. This can be ascribed to their significant characteristics, such as rapid reproduction, resilience to adverse abiotic stressors, brief generation time, target specificity, and the ability to thrive independently of their host [9-12].
Trichoderma species, particularly T. harzianum, are frequently utilized as BCAs owing to their significant antagonistic efficacy against various soil-borne plant pathogenic fungi, including Phytophthora spp., Sclerotinia spp., Rhizoctonia spp., and Fusarium spp among others [12-15]. T. harzianum exhibits the ability to inhibit pathogen growth via mycoparasitism, antibiosis, and nutrient competition in the rhizosphere [12-16], or to activate the plant’s defense mechanisms against pathogens [17]. Moreover, T. harzianum can promote plant growth and vitality when utilized alone or in combination with organic fertilizers or plant compost [18][19].
Motivated by the critical importance of maize as a major economic crop and the urgent need for environmentally friendly biocontrol methods, this study investigated the effects of T. harzianum and its combination with plant compost on promoting maize growth and mitigating root rot diseases caused by F. solani.
Materials and Methods
Fungal isolates
Fungal isolates of the pathogen F. solani were collected previously from infected corn plants. The main root of each plant sample was thoroughly rinsed, cut into 15 mm pieces, and surface sterilized using a two-step process. Root parts were immersed in 0.05% sodium hypochlorite for 1 minute, followed by immersion in 70% ethanol for 1 minute. After rinsing with sterile distilled water and air drying, three root pieces were placed on Petri dishes containing potato dextrose agar (PDA) medium and incubated at 25 ± 2°C for 7 days [2].
The biological control agent T. harzianum was isolated from agricultural soil in Hama province, Syria. The soil samples were spread onto potato dextrose agar (PDA) plates containing a small amount of streptomycin sulfate to prevent bacterial growth. These plates were incubated at 25°C for a week, allowing the fungi to grow. After this period, individual fungal colonies were carefully selected and purified by isolating single spores. These purified colonies were then incubated at 26°C for another week to ensure their purity [20]. The identification of the isolated fungi was based on their morphological traits and microscopic analysis [21]. The identification of the isolates was confirmed at the Plant Pathology laboratory at the College of Agricultural Engineering (Al-Baath University, Syria).
Preparation of fungal inoculum
T. harzianum and F. solani were prepared following the prescribed methodology of Cappuccino and Sherman [22]. The fungus was cultured in Czapek Dox medium and put on a shaker at a temperature of 25°C for 7 days. Subsequently, the resulting culture was filtered through cheesecloth to effectively separate mycelial fragments. These fragments were then thoroughly centrifuged (10,000 rpm for 15 min). The resultant suspension was subsequently adjusted, employing the plate count technique, to approximately 5×10+7 spore/ml. Following this, decimal dilutions (10-2 and 10-1) using sterilized distilled water were prepared to achieve 5×10+5 spore/ml (T1) and 5×10+6 spore/ml (T2) concentrations, respectively.
Dual culture antagonistic test
The in vitro antagonistic activity of T. harzianum against F. solani was evaluated through the implementation of the dual culture technique [23]. A 6-millimeter disk containing a pure T. harzianum culture was placed on the PDA, at 10 millimeters from the edge of the Petri dish. Another 6-millimeter disk of F. solani culture was placed on the opposite side of the dish at an equal distance. The control treatment involved inoculating a culture disk of either pathogenic or antagonistic culture individually, under the same conditions. The Petri dishes were incubated at a temperature of 25±2°C. The radial growth of F. solani colony was measured after incubation periods of 4 and 7 days. This procedure was repeated three times, and the inhibition percentage was determined by calculating the percentage of reduction in F. solani colony diameter growth in comparison to the control using the following formula:
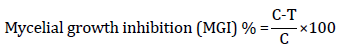
Where C is the diameter of the pathogen (F. solani) colony in control (in the absence of Trichoderma), and T is the diameter of the pathogen colony in the presence of T. harzianum [23].
Source of plant compost
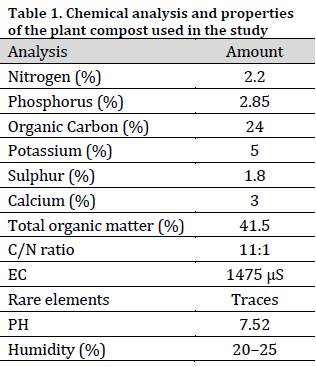
Plant compost was obtained from a commercial producer (Al-Sahel Olive Services Company, Syria). The plant compost was prepared from the remnants of potato, tomato, eggplant, olive, bitter orange, and Ficus tree. The chemical composition of compost was analyzed [24][25] in the Soil Chemistry and Fertility Laboratory at the Faculty of Agricultural Engineering, Al-Baath University, Syria (Table 1).
In planta antagonistic activity
The greenhouse experiment was conducted at the Biological Control Center in Hama province, Syria. For the in planta antagonistic assay, the native clay-loam soil was collected from a local agriculture field in Hama province. Then, it was autoclaved at a temperature of 121 °C for a duration of 1 hour to eliminate the microbiome, weeds, and nematodes. The current study examined four treatments, in addition to positive and negative F. solani controls, as follows:
- Sterilized soil as negative control (C).
- Soil infested with F.solani as positive control (P).
- Soil infested with F.solani and 5×10+5 T. harzianum (P+T1).
- Soil infested with F.solani and 5×10+6 T. harzianum (P+T2).
- Soil infested with F.solani, T. harzianum at5×10+5 , and plant compost (P+T1+Co).
- Soil infested with F.solani, T. harzianum at 5×10+6 , and plant compost (P+T2+Co).
Plastic pot with dimensions of 20×20×25 cm and filled with 5 kg of sterilized clay-loam soil were infested with a spore suspension (5×10+6) of F. solani at a rate of 50 ml per pot, 20 days prior to sowing the maize seeds [26][27]. Subsequently, 50 ml of T. harzianum spore suspension was inoculated into the soil at a concentration of 5×10+5 spore/ml (T1), or 5×10+6 spore/ml (T2) five days after F. solani inoculation. In the treatments involving plant compost (Co), a total of 100g of the plant compost was added to each pot. Treatments and controls were replicated nine times and distributed using a completely randomized design. The experiment was conducted inside the greenhouse, where the ambient temperature was maintained at 25±2 °C and the moisture level varied from 65% to 75%. The maize seeds (CASPER F1) were surface sterilized through immersion in a solution of 70% ethanol for a duration of 2 minutes, followed by exposure to a solution of 0.2% sodium hypochlorite (NaOCl) for a duration of 3 minutes. Subsequently, the seeds were subjected to multiple washes using sterile distilled water [28]. The seeds were then placed in the soil at a depth of 2–3 cm, with a planting density of 2-3 seeds per pot. The density of seedlings was subsequently reduced to 1 seedling per pot. The pots were watered every other day to maintain a field capacity level of 75%. After 75 days, the plants were carefully uprooted from the soil, and the roots were thoroughly cleaned. Plant height and wet weight of shoots and roots were measured. The plant material was placed in an oven at 60 °C for 72 hours to determine the dry weight of shoots and roots.
Re-Isolation of F. solani from maize plant
To assess the establishment of Fusarium solani endophytes within maize plants after greenhouse trials, the fungus was re-isolated from the roots of corn plants using the procedures outlined in the fungal isolates section [2].
Statistical study
One-Way Analysis of Variance (ANOVA) and means comparison via Tukey HSD test at a significance level of 0.05 was conducted of SPSS software.
Results
Dual culture assay
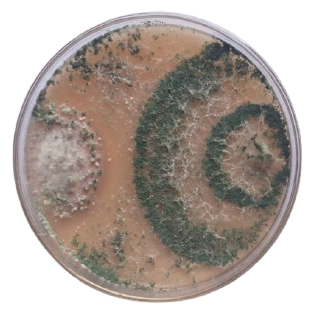
The antagonistic interaction between the pathogen F. solani and the antagonist T. harzianum was evaluated in vitro. T. harzianum mycelium rapidly overgrew the pathogen’s mycelium, preventing it from developing (Fig. 1). It was noted that T. harzianum suppressed the radial mycelial development of F. solani with mycelial growth inhibition percentages (MGI %) reaching 55% and 81% after 4 and 7 days, respectively.
Greenhouse experiment
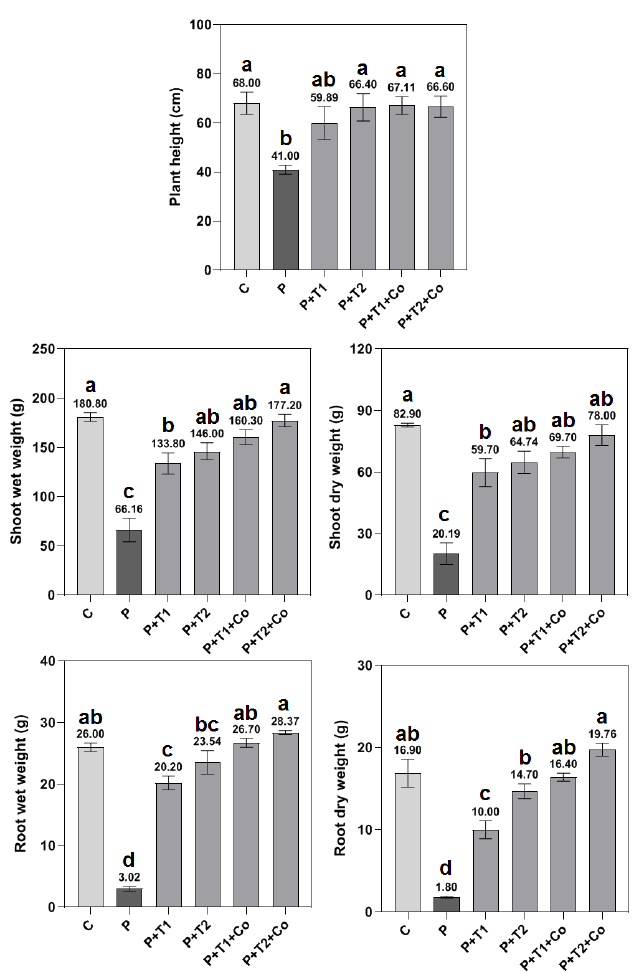
The effects of T. harzianum alone or in combination with plant compost on the growth parameters of maize plants infested with F. solani were examined. There were notable variations in the growth characteristics between the pathogen treatment (P) and other treatments. The two antagonist concentrations (5×10+5 and 5×10+6 spore/ml) utilized in treatments P+T1 and P+T2 significantly outperformed the positive infested control treatment (P) in terms of growth parameters. Despite both P+T1 and P+T2 treatments scored lower growth values compared to negative healthy control (C), this difference was not significant in P+T2 (Fig. 2). The observations demonstrate that the application of T. harzianum was effective in inhibiting disease progression or substantially mitigating its adverse effects during the initial growth phase of maize plants.
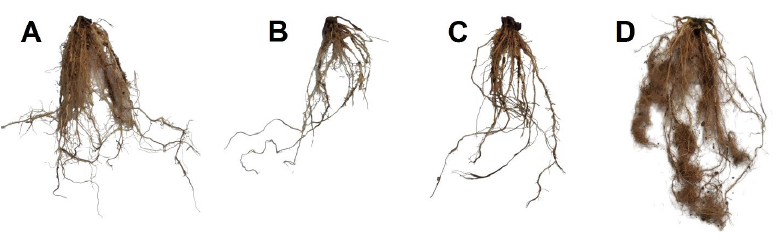
The combination of T. harzianum at 5×10+6 spores/ml and plant compost (P+T2+Co) demonstrated superior growth parameters, even surpassing those of healthy plants (C) in terms of root wet and dry weights (Figs 2 and 3). Additionally, an increased number of fine roots was noted in the P+T2+Co treatment (Fig. 3 D). Compost-supplemented treatments (P+T1+Co and P+T2+Co) surpassed the standalone T. harzianum treatments (P+T1 and P+T2) regarding the dry weight of shoots and roots.
Re-Isolation of F. solani from maize plant
The evaluation of the establishment of F. solani entophytes in maize plants was conducted upon the end of the greenhouse trials. The fungi were successfully re-isolated from all plants growing in infested soils.
Discussion
Efforts have been made over the last few decades to enhance the utilization of integrated pest management techniques and reduce reliance on agrochemicals. BCAs, thus far, constitute one of the most significant strategies employed to achieve these objectives [6][10]. These microorganisms, from bacterial and fungal origins such as bacillus [29], agrobacterium [30], and Trichoderma [5], can impede the development of plant diseases by engaging in competition for nutrients and space or producing metabolites that inhibit the growth of the pathogen [10][31]. Among these agents, Trichoderma species occupy a prominent position in the management of soil-borne pathogens [31][32]. T. atrobrunneum and T. simmonsii have demonstrated a remarkable capacity to suppress the growth and development of Rhizoctonia solani and Fusarium oxysporum f. sp. lycopersici [33]. Similarly, T. harzianum has exhibited a high efficacy against Sclerotium rolfsii, Rhizoctonia solani [34], Fusarium graminearum (a soil-borne pathogen responsible for Fusarium stalk rot in maize crops) [35], Fusarium verticillioides [36], and Fusarium root rot caused by F. solani in olive trees [37], and tomatoes [38]. Furthermore, T. harzianum has also exhibited significant antagonist activity against peanut brown root rot caused by F. solani [39].
This study demonstrated the significant in vitro antagonistic effect of T. harzianum against F. solani. T. harzianum exhibited considerable efficacy in suppressing pathogen development, as evidenced by the dual culture assay, which recorded an inhibition rate of 81%. This observation corresponds with previous reports which documented the in vitro antagonistic properties of T. harzianum against F. solani f.sp. pisi [31]. In another, T. harzianum inhibited the mycelial growth of F. solani by 51.4% in a dual culture assay [38]. This observed antagonistic effect can be attributed to various strategies possessed by T. harzianum such as parasitizing other fungi, producing antifungal substances, and competing for vital resources like nutrients and space [40][41]. A previous study showed that T. harzianum can invade and lysis the fungal hyphae of F. proliferatum and F. verticillioides, underscoring its potential for managing these pathogens [40].
The utilization of composts for the mitigation of organic waste pollution and the reduction of the application of chemical fertilizers and fungicides is a promising technique in the field of sustainable agriculture. This research showed that the combination of plant compost with T. harzianum can significantly enhance the growth parameters of the plant infested with F. solani demonstrating a notable degree of pathogen control in comparison infested plants (P) (Fig. 2). The research indicated that both concentrations of the antagonist T. harzianum (5×10+5 spore/ml and 5×10+6 spore/ml) markedly improved plant growth, as evidenced by plant height and the wet and dry weights of roots and shoots, in comparison to the positive infested control group (P). This suggests that standalone T. harzianum application can effectively prevents disease development.
The combination of T. harzianum and plant compost treatments resulted in a significant improvement in the studied growth parameters compared to the standalone T. harzianum treatments and even to the healthy plant control (C), particularly regarding the wet and dry weights of roots, along with a noticeable increase in the ratio of fine roots. According to a study, bio-compost and T. harzianum-treated soil reduced the disease incidence and severity of F. solani [26]. The combination of composted rice husks and T. harzianum showed high potential for increasing wheat shoot length and branch count per plant [18]. The benefits of using T. harzianum might be associated with either direct or indirect interaction with the pathogen; hence, T. harzianum might produce extracellular hydrolytic enzymes or toxic metabolism that effectively inhibits the pathogen development [9]. Furthermore, T. harzianum might promote the growth of maize plants, particularly when mixed with plant compost, by activating the plants’ defensive systems or by secreting gibberellic and indole acetic acids [9][42][43].
Conclusion
This study demonstrated the impact of two levels of T. harzianum, either alone or in combination with plant compost on the development of F. solani and the growth of maize plants. In both the in vitro and greenhouse experiments, the antagonist exhibited significant pathogen-controlling action. The combination of T. harzianum in both levels with plant compost resulted in higher maize plant growth parameters than other treatments. The results illustrated the significance of natural and biological materials, including organic fertilizers and biocontrol agents, in integrated pest management and in diminishing dependence on chemical pesticides and fertilizers. Further research is necessary to evaluate the antagonistic activity of T. harzianum across different crops and under field conditions.
References
- Okello PN, Petrović K, Kontz B, Mathew FM. Eight species of Fusarium cause root rot of corn (Zea mays) in South Dakota. Plant Health Prog. 2019;20(1):38–43. DOI
- Okello PN, Mathew FM. Cross pathogenicity studies show South Dakota isolates of Fusarium acuminatum, F. equiseti, F. graminearum, F. oxysporum, F. proliferatum, F. solani, and F. subglutinans from either soybean or corn are pathogenic to both crops. Plant Health Prog. 2019;20(1):44–9. DOI
- Kolomiets TM, Kiseleva MIMI, Zhemchuzhina NS, Pankratova LF, Elizarova SA. A characteristic of the species composition of pathogenic fungi of the genus Fusarium in corn biocenoses of the Voronezh region. Vav. J. Genet. Breed. 2022;26(6):583-92. DOI
- Baker BP, Green TA, Loker AJ. Biological control and integrated pest management in organic and conventional systems. Biol. Cont. 2020;140:104095. DOI
- Poveda J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol. Cont. 2021;159:104634. DOI
- He DC, He MH, Amalin DM, Liu W, Alvindia DG, Zhan J. Biological control of plant diseases: An evolutionary and eco-economic consideration. Pathogens. 2021;10(10):1311. DOI
- Niu B, Wang W, Yuan Z, Sederoff RR, Sederoff H, Chiang VL, Borriss R. Microbial interactions within multiple-strain biological control agents impact soil-borne plant disease. Front. microbiol. 2020;11:585404. DOI
- Ali A, Zeshan MA, Mehtab M, Khursheed S, Mudasir M, Abid M, Mahdi M, Rauf HA, Ameer S, Younis M, Altaf MT. A comprehensive note on Trichoderma as a potential biocontrol agent against soil borne fungal pathogens: a review. Plant Prot. 2021;5(3):171-196. DOI
- Thambugala KM, Daranagama DA, Phillips AJ, Kannangara SD, Promputtha I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. cell. infect. microbiol. 2020;10:604923. DOI
- Handelsman J, Stabb EV. Biocontrol of soilborne plant pathogens. Plant Cell. 1996;8(10):1855. DOI
- Sánchez-Montesinos B, Diánez F, Moreno-Gavíra A, Gea FJ, Santos M. Role of Trichoderma aggressivum f. europaeum as Plant-Growth Promoter in Horticulture. Agronomy. 2020 Jul 13;10(7):1004. DOI
- Adnan M, Islam W, Shabbir A, Khan KA, Ghramh HA, Huang Z, Chen HY, Lu GD. Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb. Pathog. 2019;129:7–18. DOI
- Misra V, Ansari M.I. Role of Trichoderma in agriculture and disease management. Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management. Springer. 2021; 425–40. DOI
- López-Mondéjar R, Ros M, Pascual JA. Mycoparasitism-related genes expression of Trichoderma harzianum isolates to evaluate their efficacy as biological control agent. Biol. Cont. 2011; 56(1):59-66. DOI
- De Azevedo PF, de Almeida AC, Marques RD, da Costa CL, Benedetti AR, Lescano LEAM, Matsumoto LS. In vitro inhibition of Fusarium solani by Trichoderma harzianum and biofertilizer. Res. Soc. Dev. 2021; 10(3):e5210312994-e5210312994.
- Gautam RL, Naraian R. Trichoderma, a factory of multipurpose enzymes: cloning of enzymatic genes. Fungal Biotechnol. Bioeng. 2020:137–62. DOI
- Yuan M, Huang Y, Ge W, Jia Z, Song S, Zhang L, Huang Y. Involvement of jasmonic acid, ethylene and salicylic acid signaling pathways behind the systemic resistance induced by Trichoderma longibrachiatum H9 in cucumber. BMC genomics. 2019;20:1–13. DOI
- Al-Sharmani HR, Al-Kalabi HH, AL-Abedy AN. Efficacy of rice husks compost and Trichoderma harzianum on Rhizoctonia solani and its effect on seeds germination and seedling health. In IOP Conference Series: Earth and Environmental Science. IOP Publishing. 2019;388(1):012002. DOI
- Blaya J, López-Mondéjar R, Lloret E, Pascual JA, Ros M. Changes induced by Trichoderma harzianum in suppressive compost controlling Fusarium wilt. Pestic. Biochem. Physiol. 2013;107(1):112–19. DOI
- Iqbal S, Ashfaq M, Malik AH, Khan K, Mathew P. Isolation, preservation and revival of Trichoderma viride in culture media. J. Entomol. Zool. Stud. 2017;5(3):1640-6.
- Abd El-Aziz AR, Al-Othman MR, Mahmoud MA, Metwaly HA. Biosynthesis of silver nanoparticles using Fusarium solani and its impact on grain borne fungi. Dig. J. Nanomater. Biostruct. 2015;10(2):655-62.
- Cappuccino GJ, Sherman ND. Microbiology. A laboratory Manual 4th ed. The Bejamin.1996.
- Morton DJ, Stroube WH. Antagonistic and stimulatory effects of soil microorganisms upon Sclerotium rolfsii. Phytopathology. 1955;45(8):417–20.
- Gupta, BK. Soil, plant, water and fertilizer analysis. Agrobios (India). 2007.
- Ramamoorthi V, Meena S. Quantification of soil organic carbon-Comparison of wet oxidation and dry combustion methods. Int. J. Curr. Microbiol. Appl. Sci. 2018;7(10):146-54. DOI
- Hammam MM, El-Mohamedy RS, Abd-El-Kareem F, Abd-Elgawad MM. Evaluation of soil amended with bio-agents and compost alone or in combination for controlling citrus nematode Tylenchulus semipenetrans and Fusarium dry root rot on Volkamer lime under greenhouse conditions. Int. J. ChemTech Res. 2016;9(7):86–96.
- El-Mohamedy RS, Abdel-Kader MM, Abd-El-Kareem F, Abd-Allah MA, El-Mougy NS, El-Gamal NG and Fatouh YO. Field application of bio compost to control Fusarium dry rot disease of potato in newly Reclaimed lands. J. Agric. Technol. 2012;8(4):1375–87.
- Akladious SA, Abbas SM. Application of Trichoderma harziunum T22 as a biofertilizer supporting maize growth. Afr. J. Biotechnol. 2012; 11(35): 8672–83.
- Morsy EM, Abdel-Kawi KA, Khalil MNA. Efficiency of Trichoderma viride and Bacillus subtilis as biocontrol agents against Fusarium solani on tomato plants. Egypt. J. Phytopathol. 2009;37(1):47–57.
- Penyalver R, Vicedo B, López MM. Use of the genetically engineered Agrobacterium strain K1026 for biological control of crown gall. Eur. J. Plant Pathol. 2000;106:801–10. DOI
- Kim TG, Knudsen GR. Relationship between the biocontrol fungus Trichoderma harzianum and the phytopathogenic fungus Fusarium solani f. sp. pisi. Appl. Soil Ecol. 2013; 68:57-60. DOI
- Ferreira FV, Musumeci MA. Trichoderma as biological control agent: Scope and prospects to improve efficacy. World J. Microb. Biot. 2021;37(5):90. DOI
- Natsiopoulos D, Tziolias A, Lagogiannis I, Mantzoukas S. and Eliopoulos PA. Growth-promoting and protective effect of Trichoderma atrobrunneum and T. simmonsii on tomato against soil-borne fungal pathogens. Crops. 2022;2(3):202–17. DOI
- Elad Y, Chet I, Katan J. Trichoderma harzianum: A biocontrol agent effective against Sclerotium rolfsii and Rhizoctonia solani. Phytopathology. 1980;70(2):119–21.
- Saravanakumar K, Li Y, Yu C, Wang QQ, Wang M, Sun J, Gao JX, Chen J. Effect of Trichoderma harzianum on maize rhizosphere microbiome and biocontrol of Fusarium Stalk rot. Sci. Rep. 2017;7(1):1771. DOI
- Ferrigo D, Raiola A, Piccolo E, Scopel C, Causin RJJPP. Trichoderma harzianum T22 induces in maize systemic resistance against Fusarium verticillioides. J. Plant Pathol. 2014; 96(1).
- Amira MB, Lopez D, Mohamed AT, Khouaja A, Chaar H, Fumanal B, Gousset-Dupont, A, Bonhomme L, Label P, Goupil P, Ribeiro S. Beneficial effect of Trichoderma harzianum strain Ths97 in biocontrolling Fusarium solani causal agent of root rot disease in olive trees. Biol. Cont. 2017;110:70–8. DOI
- Bokhari NA, Perveen K. Antagonistic action of Trichoderma harzianum and Trichoderma viride against Fusarium solani causing root rot of tomato. Afr. J. Microbiol. Res. 2012; 6(44):7193-7.
- Erazo JG, Palacios SA, Pastor N, Giordano FD, Rovera M, Reynoso MM, Venisse JS, Torres AM. Biocontrol mechanisms of Trichoderma harzianum ITEM 3636 against peanut brown root rot caused by Fusarium solani RC 386. Biol. Cont. 2021;164:104774. DOI
- Yassin MT, Mostafa AAF, Al-Askar AA, Sayed SR and Rady AM. Antagonistic activity of Trichoderma harzianum and Trichoderma viride strains against some fusarial pathogens causing stalk rot disease of maize, in vitro. J. King Saud Univ. Sci. 2021;33(3):101363. DOI
- Sonkar P, Chandra R, Singh R, Kumar S. Study on management of Fusarium oxysporum through different mode of action of Trichoderma spp. Int. J. Curr. Trends Sci. Technol. 2018;8:20192-20200.
- Zhang Q, Zhang J, Yang L, Zhang L, Jiang D, Chen W, Li G. Diversity and biocontrol potential of endophytic fungi in Brassica napus. Biol. Cont. 2014;72: 98–108. DOI
- Deketelaere S, Tyvaert L, França SC, Höfte M. Desirable traits of a good biocontrol agent against Verticillium wilt. Front. Microbiol. 2017;8:1186. DOI
Cite this article:
Abbod, M., Alkhouri, I., Shahoud, R. Investigating the effects of Trichoderma harzianum and plant compost on maize growth and Fusarium root rot management under greenhouse conditions. DYSONA – Applied Science, 2025; 6(1): 30-39. doi: 10.30493/das.2024.475340

