Gülendam D. Öztunç 1; Ümit. H. Erol 1*; Bekir. B. Arpacı 1
1, Department of Horticulture, Faculty of Agriculture, Kilis 7 Aralik University, 7900, Kilis, Türkiye
E-mail:
umith.erol@kilis.edu.tr
Received: 07/08/2024
Acceptance: 26/08/2024
Available Online: 26/08/2024
Published: 01/01/2025

Manuscript link
http://dx.doi.org/10.30493/das.2024.471850
Abstract
This study examined the resistance of 22 lines derived from the KM2-11, CM334, and Sena pepper genotypes, as well as 21 lines derived from the KM2-11, PM217, and Sena pepper genotypes, to Tobacco Etch Virus (TEV). The infection caused by the TEV isolates obtained from Hatay (isolate no: 774) and Antalya (isolate no: 1002) was also investigated at the seedling, fruit set, and maturation stages in the pepper varieties W4, Florida VR2, Yolo Y, and Yolo Wonder. The proliferation of TEV in mechanically infected pepper plants was examined using Double Antibody Sandwich Enzyme Linked Immunosorbent Assay (DAS-ELISA) with TEV-specific polyclonal antiserum. The proliferation was quantified by measuring the absorption values at 405 nm. Florida VR2, which has the pvr22 allele, showed resistance to the TEV isolate 1002. Among the 43 developed lines, the line 4 was resistant to both TEV isolates, while lines 61 and 62 only displayed resistance to isolate 1002. No differences were observed in the multiplication of TEV in the pepper varieties W4, Florida VR2, Yolo Y, and Yolo Wonder inoculated at different developmental stages. Nevertheless, the multiplication of TEV isolate 774 was notably reduced during the seedling stage in comparison to other developmental stages in all genotypes. The current results emphasize the need to thoroughly study the genetic mechanisms through which pepper plants acquire resistance to various TEV isolates. Additionally, it is important to investigate the potential of integrating these newly developed resistant lines into other commercially grown pepper varieties in order to enhance the crop’s ability to withstand TEV infection.
Keywords: Tobacco Etch Virus, Pepper, Plant resistance
Introduction
Originating from South America and spreading worldwide, pepper (Capsicum annuum L.) is cultivated in many regions of Türkiye as well as globally. In Türkiye, peppers are intensively grown, especially along the western and southern coastal strips, excluding high and cold plateaus such as Erzurum-Kars. Peppers are commonly cultivated in provinces such as Kahramanmaraş, Gaziantep, Adana, and Şanlıurfa, and they play a vital role in the local economy. Pepper fruits are consumed fresh or mature and are also used in the production of crushed red pepper, paste, and dried peppers.
According to numerous researchers, peppers have been used in human nutrition for around 7,000 years, and their cultivation has been carried out for 5,000 years [1]. According to archaeological evidence [2], it has been reported that peppers originated from the Americas. Specifically, Capsicum baccatum L. was first cultivated in Peru around 2000 BC. Peppers, revered for their mystical and spiritual impact on the indigenous communities of the Aztec, Maya, and Inca civilizations, were regarded as a divine offering for an extended period [3]. The Capsicum genus, which is a part of the Solanaceae family, comprises over forty identified species [4][5]. However, only five species have been domesticated: C. annuum L., C. baccatum L., C. chinense Jacq., C. frutescens L., and Capsicum pubescens Ruiz & Pav.
Virus infections are a significant factor limiting pepper cultivation. In many regions where pepper is cultivated in Türkiye, various virus infections have been reported [6-8]. Tobacco Etch Virus (TEV), a highly destructive Potyvirus, has been documented in several countries including the USA, Mexico, Sudan, Nigeria, Venezuela, and China [9]. In the Mediterranean basin, TEV has only been observed in the Southeastern Anatolia and Eastern Mediterranean regions of Türkiye [7][10]. The damage effect of TEV varies depending on the virus strain, the genetic structure of the plant, and whether the plant is infected with other viruses. According to reports, this damage has been observed to result in yield losses of up to 70% [9].
Virus infections have been widely detected in areas where peppers are cultivated, especially in open fields. A previous study detected virus infections transmitted by aphids in peppers in the Southeastern (Şanlıurfa, Gaziantep) and Eastern Mediterranean (Kahramanmaraş, Hatay) regions where pepper cultivation is widespread [7]. Collected samples were analyzed for Cucumber Mosaic Virus (CMV), Alfalfa Mosaic Virus (AMV), Potato Virus X (PVX), Potato Virus Y (PVY), Pepper Mild Mottle Virus (PepMoV), and Tobacco Etch Virus (TEV), and it was determined that 64.8% of the samples were infected with one or more viruses. Controlling the infections of PVY and TEV in pepper cultivation is possible by providing genetic resistance that is extensively studied in pepper genetic resources. PVY and TEV belong to the genus Potyvirus in the family Potyviridae, the largest family within the plant virus realm. These viruses are transmitted by aphids in a non-persistent manner. PVY, while causing widespread infections in potatoes, also causes economically significant infections in peppers, tomatoes, and tobacco, whereas TEV causes diseases in peppers, as well as in tobacco and tomatoes. The most common source of resistance in peppers is provided by the wide variation in alleles at different loci of recessive resistance genes (pvr), which offer the most effective control mechanism against different isolates of PVY and TEV [11][12].
In recent years, the impact of TEV has been increasing globally and in Türkiye. Consequently, multiple pepper breeding programs are currently underway to discover new pepper varieties that are resistant to fungal and viral infections, including TEV. The objective of this study is to identify pepper varieties that are resistant to the virus in one of the pepper breeding programs. Additionally, the study aims to determine the virus’s multiplication at various stages of pepper plant development.
Materials and Methods
Plant material
This study utilized two cultivated pepper populations, in addition to Sena and Charleston varieties, and pepper line 46. furthermore, four pepper genotypes with distinct pvr alleles [Yolo Y (PM162), Yolo Wonder (PM031), Florida VR2 (PM604), and W4], along with the Samsun tobacco variety, were employed as plant material.
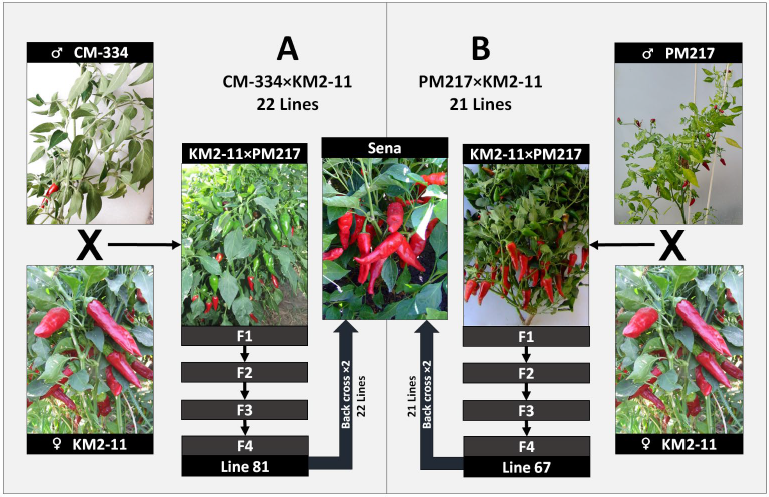
The first of the developed populations was created by crossing KM2-11 as a maternal parent (selected from the Kahramanmaraş red pepper population) with CM-334 as a paternal parent. The obtained KM2-11×CM-334 F1 progeny was self-crossed three times to obtain F4 population. Out of the obtained F4 population, line 81 was selected and backcrossed twice with the Sena red pepper variety to obtain the first experimental population of 22 lines (Fig. 1 A).
The second of the developed populations was created by crossing KM2-11 as a maternal parent with PM-217 as a paternal parent. The obtained KM2-11×PM217 F1 progeny was self-crossed thrice to obtain F4 population. Out of this population, line 67 was backcrossed twice with Sena red pepper to achieve a population consisting of 21 lines (Fig. 1 B). The lines selected from both populations were chosen due to their resistance to root rot (Phytophthora capsica) (data not shown).
Four pepper varieties, namely Yolo Wonder (PM031), Yolo Y (PM162), Florida VR2 (PM604), and W4, were selected as indicator plants to study the effects of TEV isolates and examine viral infection during three different developmental stages. The varieties possess the pvr2+, pvr21, pvr22, pvr23 alleles, respectively, which confer resistance to viral infections [13]. Furthermore, “Samsun” tobacco (Nicotiana tabacum L.) plants were used to multiply TEV isolates for use in mechanical inoculation.
Viral material
In the study, Tobacco Etch Virus (TEV) isolates numbered 774 and 1002, collected from Hatay and Antalya provinces, were used.
Seedling cultivation
The seeds of the pepper lines used in the study were sown in a 3:1 v/v peat/perlite mixture and kept in a climate chamber (24°C temperature, 16-hour photoperiod, 60% relative humidity, 3000 lux light intensity). A total of 1161 seedlings were grown from KM2-11×CM-334 and KM2-11×PM217 populations (43 lines), with 27 seedlings from each line. Furthermore, 54 control plants of varieties susceptible to TEV (Sena and Charleston pepper varieties and line 46) were cultivated with 18 seedlings from each. As for each of the pepper genotypes Yolo Wonder (PM 031), Yolo Y (PM 162), Florida VR2 (PM 604), and W4, 36 seedlings were grown, making a total of 144 seedlings.
Control of TEV isolates
The pepper genotypes Yolo Wonder, Yolo Y, Florida VR2, and W4 were each inoculated with TEV isolates separately, with 10 seedlings per genotype. The plants were monitored every two days, and symptoms were recorded.
Propagation of TEV isolates
TEV isolates obtained from the Yolo Wonder pepper genotype were introduced into Nicotiana tabacum L. “Samsun” tobacco plants using mechanical inoculation after confirming the presence of symptoms and conducting serological tests. The symptoms induced by the isolates were documented. In order to inhibit the dissemination and transmission of the isolates, chemical control measures were implemented through the regular application of pesticides targeting vectors such as aphids.
The isolates propagated in the tobacco plants were verified with the Double Antibody Sandwich Enzyme Linked Immunosorbent Assay (DAS-ELISA) using TEV-specific polyclonal antiserum before being mechanically inoculated into the pepper lines. Briefly, ELISA plates were coated with TEV polyclonal antisera, and the reading of absorbance was taken at 405 nm in a spectrophotometer after different incubation, washing, placement of the sample, adding the antiserum-enzyme conjugate, and finally applying the substrate. The method reported by [14] was used for the preservation of the isolates. As per this procedure, the isolates are initially introduced into tobacco plants. Subsequently, the infected tobacco leaves are extracted from the veins, meticulously sliced with a sharp blade, enveloped in absorbent paper, and preserved in sealed tubes containing calcium chloride.
Mechanical inoculation
Mechanical inoculation was performed separately for 774 and 1002 isolates on the cotyledon leaves of the pepper seedlings of the 43 lines and controls when the first true leaves appeared. The inoculation followed the protocol specified by [15]. The inoculum was prepared by grinding infected leaf samples in a sterile porcelain mortar with four volumes of 0.03 M phosphate buffer (Na2HPO4.2H2O-NaH2PO4.12H2O pH 7.0), 2% (w/v) DIECA, 200 mg/ml activated charcoal, and 200 mg/ml carborundum (600 mesh). The prepared inoculum was then applied to the cotyledon leaves of the plants. During the individual inoculation of each pepper seedling, special attention was given to sterilization. The inoculated plant leaves were washed with tap water after waiting for three minutes.
Similarly, both TEV isolates were mechanically inoculated into the pepper genotypes Yolo Y (PM162), Yolo Wonder (PM031), Florida VR2 (PM604), and W4 at the seedling, fruit set, and maturation stages in order to investigate TEV infection degree at different developmental stages.
Plants that showed no TEV infection symptoms and gave negative values in the first ELISA test 30 days after first inoculation were reinoculated mechanically to confirm inoculation and resistance.
DAS-ELISA assay
Leaf samples were collected from the pepper plants on the 30 days post inoculation for the selected pepper populations (the 43 lines) and controls. As for the experiment on the degree of infection during the developmental stages, leaf samples were collected on the 20 days post inoculation. The gathered samples were placed in plastic bags, marked with the line and isolate numbers, and stored at a temperature of 4°C until the ELISA test was conducted. The samples were analyzed for TEV infection using the DAS-ELISA test with TEV-specific polyclonal antiserum. The analysis was based on the readings obtained at a wavelength of 405 nm.
The necessary antisera and other materials for the ELISA (enzyme-linked immunosorbent assay) test were acquired from AGDIA, United States. The ELISA plates were coated with a diluted coating buffer at a ratio of 1:200, as instructed by the manufacturer of the TEV polyclonal antiserum. A volume of 100 µl of the TEV polyclonal antiserum was added to each well. The plates were then covered and incubated in humidity chamber storage containers at a temperature of 37°C for 2 hours. After the incubation period, the plates were rinsed with washing buffer three times, with each rinse lasting three minutes, and then dried. The leaf samples, weighing 100 mg, were pulverized with grinding buffer at a ratio of 1:10 (w:v). The pulverized samples were then stored on ice until they were ready to be used.
The prepared samples were placed in the wells of the plates in two identical sets, with each well containing 100 µl of the sample. The plates were left to incubate for 16 hours at a temperature of +4°C. The plates were rinsed with washing buffer three times at three-minute intervals and then dried. The antiserum-enzyme conjugate was diluted in the conjugate buffer according to the manufacturer’s recommendation, at a ratio of 1:200. Then, it was added to each well at a volume of 100 µl per well. The plates were placed in humidity chamber storage containers and incubated at a temperature of 37°C for a duration of 2 hours. The plates were rinsed with washing buffer three times at three-minute intervals and then dried. The p-nitrophenyl phosphate substrate was prepared in substrate buffer at a concentration of 1 mg/ml and then added to the wells at a volume of 100 µl per well. The plates were stored in darkness at ambient temperature, and the alteration in color of the wells was observed. Measurements were conducted at a wavelength of 405 nm using an ELISA plate reader at intervals of 30 minutes and 60 minutes. The ELISA assay was carried out using three biological replicates, and each biological replicate was performed with three technical replicates and the results were shown as averages of technical and biological replications.
Results and Discussion
TEV symptomatologic observations
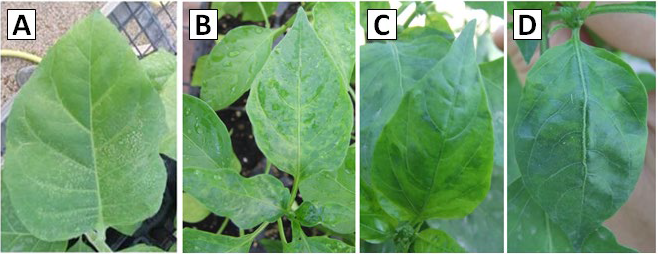
Symptoms of Tobacco Etch Virus became apparent on the plants ten days after they were mechanically infected. The symptoms exhibited variations between the initial and advanced stages. The TEV isolates induced chlorosis in the interveinal regions of young tobacco leaves and necrotic lesions in older leaves. Additionally, chlorosis was detected in the leaf veins (Fig. 2 A). As for infection symptoms on pepper plants, the isolates did not induce mosaic, banding, deformations, or necrosis in the leaves. The symptoms, initially manifested as chlorosis between the leaf veins, did not advance to necrosis in subsequent stages. The isolates, which induced chlorosis in the veins, displayed symptoms that closely resembled those observed in young tobacco plants (Fig. 2 B-D).
TEV serological tests
Responses of indicator plants and developed populations to TEV
The ELISA readings, which are below the threshold value of three times the negative control, indicate the absence of Tobacco Etch Virus (TEV) in the sample and demonstrate resistance to TEV multiplication. None of the genotypes containing the pvr locus showed resistance to TEV either isolates except for Florida VR2, which contains the pvr22allele and had a negative TEV ELISA reading (0.026) with isolate number 1002 (Fig. 3).
All developed genotypes (43 lines) were susceptible to TEV infection, except for line number 4 which showed no visual symptoms. Furthermore, this line had a negative ELISA read for TEV isolate 774 (0.023) and an extremely low read for TEV isolate 1002 (0.066). This resistance is attributed to the paternal parent CM334, which includes Pvr4 and pvr23 resistance alleles [10] and indicating an introgression of resistance genes in line 4. Compared to Florida VR2 (Fig. 3), it is thought that this genotype may have resistance loci other than the pvr22 allele.
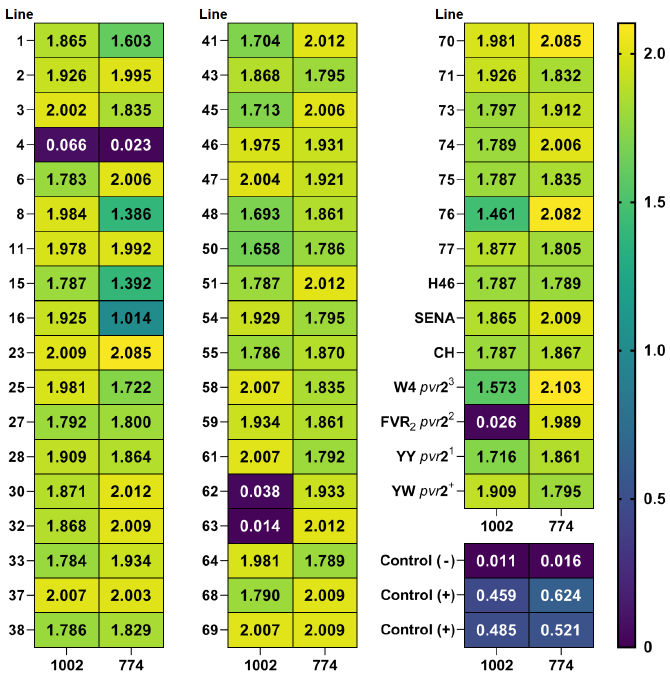
Similar to Florida VR2, lines 61 and 62 showed resistance to TEV isolate number 1002 but could not prevent the multiplication of TEV isolate number 774. Although a previous study reported a negative correlation between the multiplication and virulence of Tobacco Etch Virus isolates [16], the pathogen’s inability to multiply in the host can be interpreted as a form of resistance. This conclusion is further reinforced when considering the lack of symptoms associated with viral infection observed in line 4. Thus, it can be asserted that lines exhibiting resistance to the pathogen impede its multiplication. The inhibition of TEV multiplication in plant hosts is caused by specific mutations that occur at the pvr locus in peppers. These mutations provide resistance against TEV by preventing virus replication [11].
The previously mentioned lines (4, 61, and 62) show great potential as candidates for pepper breeding programs aimed at developing resistance to TEV. Additional investigation is required to determine the genetic mechanisms responsible for the resistance of these genotypes to TEV infection, particularly Line 4, which exhibited resistance to both 774 and 1002 isolates. It is also important to determine if these lines would display resistance to other TEV isolates found in the same area.
Multiplication of TEV isolates in peppers at different developmental stages
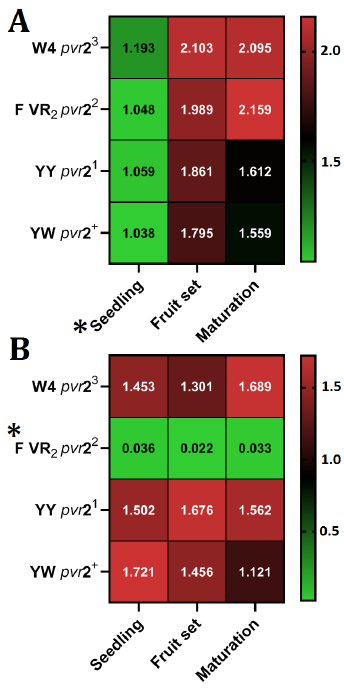
TEV isolate number 774 exhibited elevated readings in all genotypes and proliferated in all pepper genotypes across all three developmental stages. Based on the Tukey HSD test, the ELISA readings of TEV isolate 774 multiplication were significantly lower (p<0.05) at the seedling stage (average 1.0845) compared to other developmental stages across all genotypes. However, the levels of TEV isolate 774 multiplication in fruit set and maturation stages were similar (p>0.05). No statistically significant differences (p>0.05) were observed in terms of the multiplication of TEV isolate 774 between the genotypes under investigation (Fig. 4. A).
Regarding TEV isolate number 1002, there was a notable decrease (p>0.05) in multiplication levels when inoculated in the Florida VR2 pepper variety, which possesses the pvr22 allele, at all three developmental stages. However, there were no statistically significant differences (p>0.05) in the multiplication of TEV isolate 1002 between the different developmental stages (Fig. 4 B).
The pepper-TEV molecular interaction is still far from being fully understood. While eleven chromosomal regions associated with quantitative resistance to PVY have been identified, many alleles providing complete resistance to TEV have not been characterized yet [17]. It has been found that the heterozygosity of pvr2 alleles enhances resistance effectiveness against potyviruses [18][19]. It was previously reported that TEV infectivity (both highly transmitted by aphids and non-wilting isolates) can be blocked by the wild-type eIF4E in pvr1 and pvr12 [19]. On the other hand, pvr1 does not confer resistance to the Mex21 TEV isolate [20].
A previous study found that the level of Turnip Mosaic Virus in Arabidopsis thaliana plants increases as they grow older [21]. Nevertheless, it has been documented that the presence of CMV in peppers is independent of the age of the plant [22]. In another study, no correlation between the severity of symptoms and the age of the tomato plants (14, 28, and 45 days old) that were either resistant or susceptible to Tomato Yellow Leaf Curl Virus (TYLCV) [23]. However, the researchers did find a significant relationship between the age at which the plants were infected and their yield [23]. The current research demonstrated that the initial phases of plant development (specifically the seedling stage) may pose challenges to the proliferation of TEV isolate 774, in contrast to later stages. However, isolate 1002 did not show any hindering effects, indicating that different TEV isolates may have varying interactions with the pepper host depending on the plant’s growth phase.
It has been reported that the early infection of Beet Curly Top Virus in sugar can have a significant impact on resistant genotypes. As the plant matures, however, the pathogen is unable to affect the resistant varieties [24]. A minor discrepancy was observed in the current work in relation to TEV isolate 1002, as the resistant variety Florida VR2 consistently exhibited resistance throughout the entire experiment. This emphasizes that the mechanism of resistance against TEV infection is effective in resistant varieties from the early seedling phase and throughout the life of the plant.
Conclusion
Among the various types of pepper, only Florida VR2 variety exhibited resistance to the TEV isolate 1002. Line 4, which was obtained from genotypes CM334, PM217, and Sena, demonstrated resistance to both TEV isolates 1002 and 774. Additionally, lines 61 and 62 only displayed resistance to isolate 1002. Lines 4, 61, and 62, which have been identified as having resistance to TEV infection, should be subjected to molecular-level analysis in order to determine the specific genetic factors that are responsible for this resistance. The TEV virus underwent replication during every stage of pepper plant development. Nevertheless, the replication of TEV isolate 774 was notably reduced during the seedling stage in comparison to other developmental stages in all genotypes. Further research should focus on elucidating the mechanisms by which pepper plants impede TEV infection during the initial stages. Effective virus management involves the identification of the pathogen and the implementation of measures to impede its transmission, as direct chemical control is not effective. It is imperative to eradicate infected plants, control the spread of disease-carrying organisms, and implement preventive measures to avoid contamination in both enclosed cultivation spaces and open fields. More importantly, it is crucial to include promising lines, such as those reported in the current study, in pepper breeding programs to enhance resistance against TEV.
Acknowledgments
We would like to thank Kilis 7 Aralik University for providing laboratory facilities for this study. This article is based on the master thesis of the first author.
References
- Heiser B. Seed to Civilization. The story of man’s food. Freeman. San Francisco/Reading. 1973; 243.
- Pickersgill B. The archaeological record of chili peppers (Capsicum spp.) and the sequence of plant domestication in Peru. Am. Antiq. 1969;34(1):54-61. DOI
- Bosland PW, Votava EJ, Votava EM. Peppers: Vegetable and Spice Capsicums. CAB International, Wallingford. UK. 1999;204.
- Barboza GE, García CC, de Bem Bianchetti L, Romero MV, Scaldaferro M. Monograph of wild and cultivated chili peppers (Capsicum L., Solanaceae). PhytoKeys. 2022;200:1-7. DOI
- Mavi K. Biberlerde türler arası melezleme. Int. J. Life Sci. Biotechnol. 2020;3(3):386-406. DOI
- Arli-Sokmen M, Mennan H, Sevik MA, Ecevit O. Occurrence of viruses in field-grown pepper crops and some of their reservoir weed hosts in Samsun, Turkey. Phytoparasitica. 2005;33:347-58.. DOI
- Buzkan N, Demir M, Öztekin V, Mart C, Çaǧlar BK, Yilmaz MA. Evaluation of the status of capsicum viruses in the main growing regions of Turkey. EPPO Bulletin. 2006;36(1):15-9. DOI
- Buzkan N, Arpaci BB, Simon V, Fakhfakh H, Moury B. High prevalence of poleroviruses in field-grown pepper in Turkey and Tunisia. Arch. Virol. 2013;158:881-5. DOI
- Green SK, Kim JS. Characteristics and control of viruses infecting peppers: a literature review. Shanhua, Taiwan: Asian Vegetable Research and Development Center. 1991.
- Moury B, Verdin E. Viruses of pepper crops in the Mediterranean basin: a remarkable stasis. Adv. Virus Res. 2012;84:127-62. DOI
- Robaglia C, Caranta C. Translation initiation factors: a weak link in plant RNA virus infection. Trends Plant Sci. 2006;11(1):40-5. DOI
- Charron C, Nicolai M, Gallois JL, Robaglia C, Moury B, Palloix A, Caranta C. Natural variation and functional analyses provide evidence for co‐evolution between plant eIF4E and potyviral VPg. Plant J. 2008;54(1):56-68. DOI
- Arpaci BB. Distribution and genetic diversity of tobacco etch virus in Turkey and resistance of improved capsicum lines. Appl. Ecol. Environ. Res. 2019;17(6):14641-51. DOI
- Bos L, Benetti MP. Direct electron microscopy and serology with plant viruses in leaf material dried and stored over calcium chloride. Neth. J. Plant Pathol. 1979; 85(6):241-51. DOI
- Moury B, Morel C, Johansen E, Guilbaud L, Souche S, Ayme V, Jacquemond M. Mutations in Potato virus Y genome-linked protein determine virulence toward recessive resistances in Capsicum annuum and Lycopersicon hirsutum. Mol. Plant-Microbe Interact. 2004;17(3):322-29. DOI
- Lafforgue G, Martínez F, Sardanyés J, De la Iglesia F, Niu QW, Lin SS, Elena SF. Tempo and mode of plant RNA virus escape from RNA interference- mediated resistance. J. Virol. 2011; 85(19):9686-95. DOI
- Caranta C, Palloix A. Both common and specific genetic factors are involved in polygenic resistance of pepper to several potyviruses. Theor. Appl. Genet. 1996;92(1):15-20. DOI
- Moury B, Palloix A, Caranta C, Gognalons P, Souche S, Selassie KG, Marchoux G. Serological, molecular, and pathotype diversity of Pepper veinal mottle virus and Chili veinal mottle virus. Phytopathology. 2005;95(3):227-32. DOI
- Rubio M, Nicolaï M, Caranta C, Palloix A. Allele mining in the pepper gene pool provided new complementation effects between pvr2-eIF4E and pvr6-eIF(iso)4E alleles for resistance to pepper veinal mottle virus. J. Gen. Virol. 2009;90(11):2808-14. DOI
- Murphy JF, Blauth JR, Livingstone KD, Lackney VK, Jahn MK. Genetic mapping of the pvr1 locus in Capsicum spp. and evidence that distinct potyvirus resistance loci control responses that differ at the whole plant and cellular levels. Mol. Plant-Microbe Interact. 1998;11(10):943-51. DOI
- Yao Y, Kathiria P, Kovalchuk I. A systemic increase in the recombination frequency upon local infection of Arabidopsis thaliana plants with oilseed rape mosaic virus depends on plant age, the initial inoculum concentration and the time for virus replication. Front. Plant Sci. 2013:4. DOI
- Garcia‐Ruiz H., Murphy JF. Age‐related Resistance in Bell Pepper to Cucumber mosaic virus. Ann. Appl. Biol. 2001;139(3):307-17. DOI
- Levy D, Lapidot M. Effect of plant age at inoculation on expression of genetic resistance to tomato yellow leaf curl virus. Arch. Virol. 2008;153(1):171-79. DOI
- Wintermantel WM, Kaffka SR. Sugar beet performance with curly top is related to virus accumulation and age at infection. Plant Dis. 2006;90(5):657-62. DOI
Cite this article:
Öztunç, G., Erol, Ü., Arpaci, B. Investigation of Tobacco Etch Virus (TEV) multiplication at different developmental stages in pepper. DYSONA – Applied Science, 2025;6(1): 16-24. doi: 10.30493/das.2024.471850

