Sevidzem S. Lendzele 1; Mintsa-Nguema Rodrigue 1; Makanga Boris 1; Pyazzi O. O. Kutomy 1,2,3; Koumba A. Armel 1; Zinga-Koumba R. Christophe 1; Sima O. R. Leotard 1; Jacques F. Mavoungou 1
1, Laboratory of Vector Ecology, Institute Tropical Ecology Research (IRET), Libreville, Gabon
2, Cheikh Anta Diop University of Dakar (UCAD), Dakar, Senegal
3, National Malaria Control Program (PNLP), Libreville, Gabon
E-mail:
sevidzem.lendze@gmail.com
Received: 17/03/2022
Acceptance: 21/04/2022
Available Online: 23/04/2022
Published: 01/10/2022

Manuscript link
http://dx.doi.org/10.30493/DLS.2022.334581
Abstract
Malaria caused by Plasmodium falciparum is one of the most frequently diagnosed diseases in health facilities of sub-Saharan Africa. To investigate the correlation between malaria cases occurrence and the age, sex, and location of patients, a case study was conducted at the Mitzic Medical Center (MMC) in north Gabon. Malaria cases prevalence in the center was determined between 1 January to 31 December 2021. Furthermore, an entomological study was conducted in different sites of Mitzic to identify Anophelinae larval breeding habits and relate them to the frequency of reported malaria cases. Of the 765 diagnoses, 187 were positive, resulting in an overall prevalence of 24.44%. Patients from towns had a high prevalence compared to their village counterparts. Patients between 0 to 10 years had a high prevalence rate than the other age cohorts. Females had a high prevalence than males in both town and village. In the study area, 44 Anophelinae positive larval breeding sites were identified, with the majority of these sites being located in the town. Therefore, the increasing anthropic activities in town and the proximity of larval habitats to human dwellings were identified as potential infection risk factors in this area.
Keywords: Malaria, Anopheles, Breeding sites, Risk factors
Introduction
Malaria caused by Plasmodium falciparum is a parasitic disease frequently detected in patients coming for consultation in health facilities in sub-Saharan Africa. In the Central African sub-region, malaria is a public health issue where many cases are yearly reported in health facilities [1] and even in schools [2]. In Gabon, some papers have been published on malaria prevalence in health facilities [3]. However, the available information regarding the association between the demographic distribution of cases and environmental diversity is limited.
It is known that the presence of favorable breeding grounds for female Anopheles mosquitoes represents a risk for the spread of malaria. However, it is necessary to understand the correlation between infection prevalence and demographic/environmental composition. Therefore, this study attempts to investigate the correlation between affirmative malaria cases and the demographic variables of patients. Furthermore, an entomology study was carried out to associate breeding sites and cases distribution in the area. This information is crucial for the effective implementation of malaria control strategies in Mitzic.
Materials and Methods
Study area
Mitzic is the capital city of the Okano department and is 111.47 km from Oyem, the capital of the Woleu-Ntem province of Gabon. Geographically, it is located within latitude 0° 42′ 11″ north and longitude 11° 33′ 57″ East. Mitzic is one of the towns of North Gabon that are closer to the borders of Cameroon and Equatorial Guinea. The population of this town mainly depends on commerce, with most goods originating from neighboring countries and an underdeveloped agricultural sector. The population in surrounding villages are forestry workers as logging by forestry companies is common. The ongoing city development projects, deforestation, the opening of roads, and construction work in some villages by forestry companies are some of the anthropic activities that could modify the ecosystem by creating favorable breeding sites for malaria vectors.
The Mitzic Medical Center (MMC) is the only government primary health facility that receives patients from all parts of the town and surrounding villages. However, some villages have dispensaries that provide basic health care for the population.
Patients sample
A health-facility-based study regarding the patients’ age, sex, and place of residence was conducted at the MMC. All patients coming for consultation from January to December 2021 and were recommended to conduct a malaria test were included in this study. Blood collection was carried out by a well-trained laboratory technician. The laboratory test for malaria at the MMC was the rapid diagnostic test. Only the ONE STEP Malaria HRP-II (P.f) and PLDH (Pan) antigen rapid test of the SD BIO LINE Malaria Ag Pf/Pan mark is used in this medical center.
Entomological study
This study was conducted daily from 8 a.m to 2 p.m. The search for larval habitats of Anophelinae was conducted through a systematic verification of the potential mosquito larval breeding sites using the dipping method [4].
Morphological identification of Anophelinae larvae
Anopheles larvae were identified by their characteristic horizontal positioning on the surface of the water. The morphological identification of the larvae was conducted following the morphological traits [5] and the key of Gillies and Coetzee [6].
Data analysis
Statistical analysis was conducted using python 3.6. The Chi-square and the Mann-Whitney tests were used to compare the malaria prevalence with the site, sex, and age. The level of significance was stated at p<0.05 at 95 % confidence interval.
Results
Malaria prevalence
Of the 765 patients that conducted malaria tests from January to December 2021, 187 tested positive for the disease (Fig. 1), with an overall prevalence rate of 24.44%.
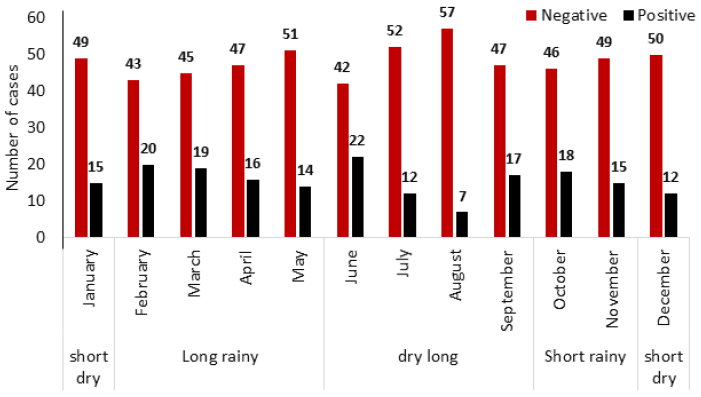
Prevalence with site
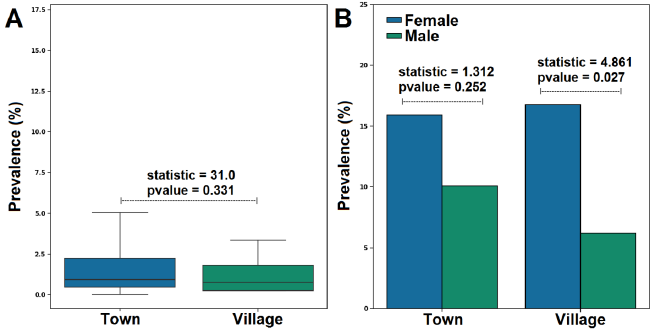
Based on the prevalence with the site, a high prevalence was recorded in patients originating from town (25.99%) than village (22.94%). However, this difference was insignificant (P=0.331) (Fig. 2 A).
Prevalence with sex
The results showed that females had a higher infection prevalence than their male counterparts in the town and the village. Interestingly, there was a statistically significant difference in prevalence rate with sex in the village but not in the town (Fig. 2 B).
Prevalence with age
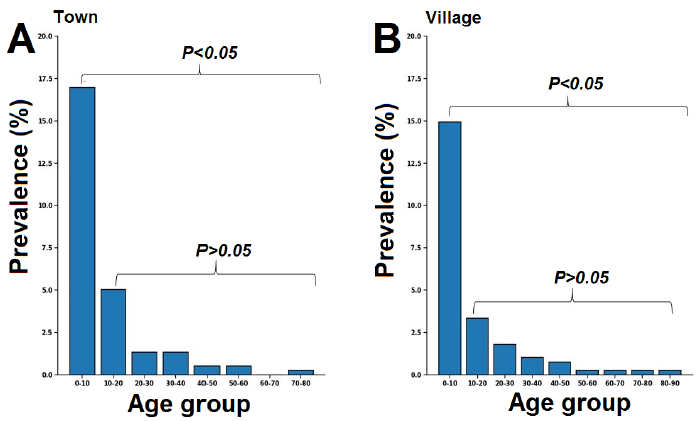
Based on prevalence with age cohort, in town as in the village, a high infection rate was found in patients aged 0 to 10 years (Fig. 3). This infection prevalence in kids (0-10 years) was significantly higher than in other age groups, while no significant differences were observed between other groups.
Larval habitats inspection
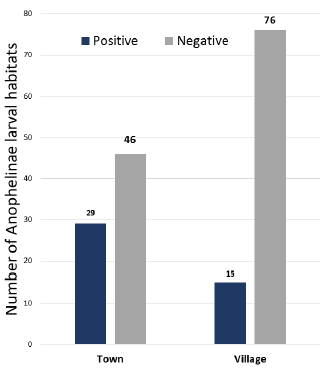
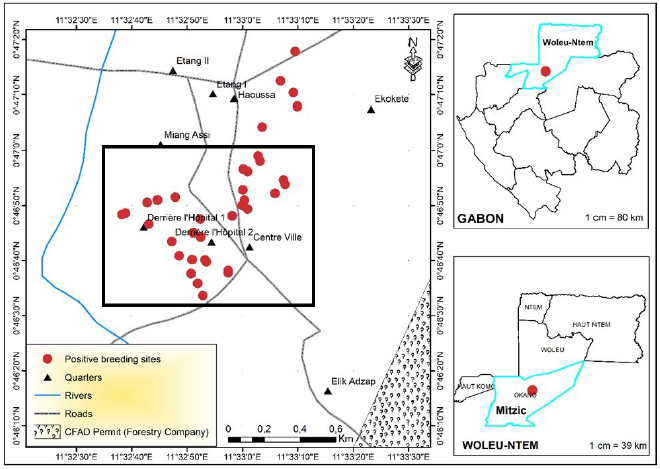
The entomological study led to the identification of 44 Anophelinae larval habitats in the town and village sites of Mitzic. Although more sites were inspected in the village, almost twice the number of Anophelinae larval habitats were identified in town compared to the surrounding villages (Fig. 4). The mapping of Anophelinae breeding sites reveals the clustering of larval habitats in the center of the town (Fig. 5). These breeding habits were found around homesteads of quarters where patients originated.
Environmental risk factors for larval development in village and town
The different anthropic activities in the city and villages of Mitzic have led to the modification of its ecosystem and consequently expanded mosquito breeding sites. The village of Mitzic is characterized by logging activities conducted by forestry companies. This activity has created jobs for villagers and is a vital source of income. The population uses the village’s river for bathing and laundry, and Anophelinae larvae were found at the edges of this water body. Most houses are poorly made from wood and have open eaves and holes, enabling the entry of mosquitoes. (Fig. 6 A). However, the local houses in some communities where forestry companies exploit local wood are modern wooden houses with close eaves and walls that do not permit the entry of biting insects, especially mosquitoes (Fig. 6 B). Moreover, forestry companies have opened roads to ease forest accessibility. The tracks created by wood transporting trucks retain water during the rainy season and form a temporal larval habitat for Anophelinae (Fig. 6 C).

The city of Mitzic, where the MMC is located, is characterized by commercial activities, and some of the prominent ones include bars and garages (Fig. 7 A). These activities modify the environment leading to the expansion of Anophelinae breeding sites. Stagnant water spots, tires, and abandoned cars were identified to harbor Anophelinae larvae around some garages (Fig. 7 A and B). After a thorough inspection of the immediate environment of human dwellings, it was found that because of the scarcity of water in the city, the population used water storage materials such as buckets, pots, drums, and containers. These open water surfaces harbor a high density of Culicinae larvae but not Anophelinae larvae (Fig. 7 A, C, and D).

Discussion
Malaria remains the most diagnosed disease in health facilities in Africa, particularly in Gabon. In this study, the overall malaria prevalence was 24.44% among the investigated potential cases, close to the 30% reported by authors from neighboring Congo [7]. A high prevalence of malaria was observed in the town compared to the village setting, which could be explained by the ongoing urbanization. The construction of roads, public buildings, and the developing commercial activities in Mitzic town have contributed to creating and expanding Anophelinae larval breeding sites compared to the underdeveloped village. On the other hand, the housing type in the village could be one of the reasons for malaria cases, as 95 % of the houses were wooden houses with holes that could permit the entry of mosquitoes. According to [8], children living in modern houses had low malaria prevalence than those living in primitive houses. Furthermore, the report of [9] in Malawi showed that the high prevalence of urban malaria is due to people’s travel from urban to rural areas.
The high infection rate among the young cohort is mainly attributed to their vulnerable immune system. The children usually play around rivers, streams, and other potential Anophelinae breeding grounds naked or partially dressed, exposing them to mosquito bites and subsequent infection. This finding is not strange as most authors have reported high cases of malaria in children in Gabon [10] [11] and elsewhere in Africa [12]. In both town and village settings, a high malaria prevalence rate was recorded in females than males. This finding could be explained by the fact that women frequently visit the hospital for anti-natal clinics. It is known that women in general and particularly pregnant women have a weak immune system due to estrous cycles and pregnancy, hence their high vulnerability to infectious diseases such as malaria. Similarly, an Ethiopian study reported higher malaria cases in women than men [12].
The identification and characterization of Anophelinae breeding substrates is a prerequisite for an effective targeted larval habitats control program [13]. In the present study, 44 positive Anophelinae larval breeding sites were identified and frequently found by river edges, open soil surfaces, abandoned cars, and containers. High diversity of these larval breeding habitats was found in the town compared to the village. The primary and permanent Anophelinae breeding site identified in the village of Mitzic was river edges, while open soil water surfaces were identified as temporal Anophelinae larval breeding sites. These breeding sites pose direct infection risks [12][14]. Most of the temporary breeding sites correlated with truck traffic activities (Fig. 6 C) and (Fig. 7 E); similarly, breeding sites were linked with human land uses, rivers, and roads in Ecuador [15].
It can be concluded that human activities can indirectly support the spread of the disease by providing a suitable temporary environment for vector breeding. However, mapping these breeding sites can give an overall idea for the application of efficient control programs. On the other hand, considering the higher infection rates in children, spreading awareness of protection measures among this age group is a must.
Ethical considerations
The protocol for the malaria vector cartography project that included malaria infections in the population was approved by the National Ethics Committee of Gabon (nº PROT 016/2019/PR/SG/CNE). An authorization (nº 096/MS/SG/DGS/DRSN/CMM) was obtained from the chief medical officer to use the data on malaria cases. Only age, sex, malaria test results, and origin of patients were anonymously included in the database.
Acknowledgements
This study was conducted with the financial support of the French Agency for Development (AFD) in collaboration with the national malaria control program (PNLP) of Gabon. We are grateful to the local population for facilitating the study.
References
| 1 | Lendzele SS, Abdoulmoumini M, Abdoulaye M. Typhoid, Malaria and Their Concurrent Infections in Fondonera, West Region of Cameroon. J. Vet. Sci. Med. Diagn. 2017;6:3. DOI |
| 2 | Bamou R, Sevidzem SL. ABO/Rhesus Blood Systems and Malaria Infection among Students of the University of Dschang-Cameroon. Malaria World J. 2016;7:4. |
| 3 | Lekana-Douki JB, Pontarollo J, Zatra R, Toure-Ndouo FS. Paludisme au Gabon : résultats d’une étude bioclinique à l’hôpital de l’amitié sino-gabonaise de Franceville. Sante. 2011;21:193-8. DOI |
| 4 | Silver JB. Mosquito ecology: field sampling methods. springer science & business media; 2007. |
| 5 | Sallum MAM, Obando RG, Carrejo N, Wilkerson Richard C. Identifcation keys to the Anopheles mosquitoes of South America (Diptera: Culicidae). II. Fourth-instar larvae. Parasites Vectors. 2020;13:582. DOI |
| 6 | Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Publication of the South African Institute for Medical Research. 1987;55:1–143. |
| 7 | Deutsch-Feldman M, Brazeau NF, Parr JB. Spatial and epidemiological drivers of Plasmodium falciparum malaria among adults in the Democratic Republic of the Congo. BMJ Glob. Health. 2020;5:e002316. DOI |
| 8 | Tusting LS, Bottomley C, Gibson H, Kleinschmidt I, Tatem AJ, Lindsay SW. Housing Improvements and Malaria Risk in Sub-Saharan Africa: A Multi-Country Analysis of Survey Data. PLoS Med. 2017;14:e1002234. DOI |
| 9 | Mathanga DP, Tembo AK, Mzilahowa T, Bauleni A, Mtimaukenena K, Taylor TE, Valim C, Walker ED, Wilson ML. Patterns and determinants of malaria risk in urban and peri-urban areas of Blantyre, Malawi. Malar. J. 2016;15:590. DOI |
| 10 | Maghendji-Nzondo S, Nzoughe H, Lemamy GJ, Kouna LC, Pegha-Moukandja I, Lekoulou F, Mbatchi B, Toure-Ndouo F, Lekana-Douki JB. Prevalence of malaria, prevention measures, and main clinical features in febrile children admitted to the Franceville Regional Hospital, Gabon. Parasite. 2016;23:32. DOI |
| 11 | Pegha MI, Biteghe Bi Essone JC, Sagara I, Kassa Kassa RF, Ondzaga J, Lékana Douki J-B. Marked Rise in the Prevalence of Asymptomatic Plasmodium falciparum Infection in Rural Gabon. PLoS ONE. 2016 ;11:e0153899. DOI |
| 12 | Alemu A, Wondewosen T, Golassa L, Abebe G. Urban malaria and associated risk factors in Jimma town, south-west Ethiopia. Malar. J. 2011;10:173. DOI |
| 13 | Kweka EJ, Zhou G, Munga S, Lee M-C, Atieli HE. Anopheline Larval Habitats Seasonality and Species Distribution: A Prerequisite for Effective Targeted Larval Habitats Control Programmes. PLoS ONE. 2012;7:e52084. DOI |
| 14 | Aklilu E, Kindu M, Gebresilassie A, Yared S, Tekie H, Balkew M. Environmental factors associated with larval habitats of anopheline Mosquitoes (Diptera: Culicidae) in Metema District, Northwestern Ethiopia. J Arthropod Borne Dis. 2020;14(2):153- 61. DOI |
| 15 | Pinault LL, Hunter FF . Larval habitat associations with human land uses, roads, rivers, and land cover for Anopheles albimanus, A. pseudopunctipennis, and A. punctimacula (Diptera: Culicidae) in coastal and highland Ecuador. Front. Physiol. 2012;3:59. DOI |
Cite this article:
Lendzele, S., Rodriquge, M., Boris, M., Kutomy, P., Armel, K., Christophe, Z., Leotard, S., Mavoungou, J. Is malaria cases frequency correlated with the environmental and demographic composition at Mitzic medical center in Gabon?. DYSONA – Life Science, 2022;3(2): 41-48. doi: 10.30493/dls.2022.334581
