Monica O. Oguntimehin 1; Bartholomew S. Adeleke 1*
1, Department of Biological Sciences, Olusegun Agagu University of Science and Technology, Okitipupa, Nigeria
E-mail:
microbade@yahoo.com
Received: 04/11/2021
Acceptance: 09/01/2022
Available Online: 12/01/2022
Published: 01/07/2022

Manuscript link
http://dx.doi.org/10.30493/DAS.2022.313488
Abstract
This study was designed to evaluate the microbiological, physical, and chemical effects of ginger extracts and sterilization on palm oil samples sourced from markets in the Southern Senatorial District of Ondo State, Nigeria. Water and ethanol ginger extracts were used for palm oil treatment. Microbial isolation was performed by serial dilution and pour plating techniques. Pure isolates were obtained by repeated streaking. Isolates were identified based on cultural, biochemical, and morphological characterization. Biochemical parameters, such as free fatty acid, peroxide value, moisture content, impurity level, and iodine value, were determined. Sterilized palm oil had no microbial growth with low values in all evaluated parameters. The bacterial genera isolated from the palm oil samples included Pseudomonas, Bacillus, Enterococcus, Staphylococcus, and Micrococcus. The discovered fungal species included Aspergillus niger, A. flavus, Cochliobolus sp., Penicillium citrinum, P. italicum, P. chrysogenum, Geotrichum clavatum, Mucor spp., Chrysosporium tropicum, Fusarium solani, Microsporum canis, and Meyerozyma guilliermondii. These microorganisms indicated the unhygienic condition of palm oil production chain from processing to packaging and market display. Both ginger extracts and sterilization were efficient in combating oxidative rancidity of palm oil and controlling microbial growth that might alter oil quality. Therefore, adequate quality control measures of palm oil during processing and marketing and treatments will help to reduce post-production contamination and deterioration under storage conditions.
Keywords: Palm oil, Zingiber officinale, Oil contamination, Free fatty acids, Peroxide value
Introduction
Oil palm (Elaeis guineensis) is an ornamental plant found growing in a warm climate at high altitudes less than 1,600 feet above sea level [1]. The plant is commonly found in the arid and semi-arid regions with many peculiarities to West Africa and other countries in the world [2]. The palm fruits are usually harvested from bunches and processed to produce palm oil upon maturation. Palm oil is reddish and contains high pigments content, such as beta-carotene, alpha-carotene, and lycopene [3]. Similar to other oilseed crops, palm oil contains high fiber, debris, saturated and monounsaturated fatty acid, oleic acid, fatty acid, palmitic acid, glyceryl laurate, myristate, palmitate, stearate, oleate, linoleate, and linolenate [4][5]. Palm oil has been globally recognized as an edible oil based on its nutritional value [6]. Out of the ten major oilseeds, oil palm accounts for 5.5% of global land use for cultivation while producing 32% of global oils and fats output and 40% share of the world’s edible oils trading volume [7].
The use of palm oil in food production and industries is known [8][9]; however, harnessing its full potential in food industries as a composite can enhance dietary food value for human nutrition. Additionally, similar to other seed oils, palm oil nutritional and health benefits have been documented in the literature [5-10].
In Nigeria, the demand for palm oil needs to be intensified to meet human needs and industrial use. Thus, many rural dwellers engage in palm oil processing using a tedious manual approach [11]. Some palm oil processors apply little or no hygiene procedures [12]. Nevertheless, proper hygiene measures are needed to create awareness of quality control and hygienic practices in producing quality palm oil. Speculatively, the potentially harmful effects of unrefined traditionally processed palm oil can outweigh its nutritional benefits due to the presence of some components, which contribute to the numerous chemical reactions in the degradation of such product [13]. In Nigeria, purchased or processed palm oil is usually stored in big containers for several months or years for economic reasons. Most importantly, buying palm oil in large quantities for domestic use or by merchants does arise during the high production time (April-July), when the price is low and later sold at a high price after six months of harvesting. However, one of the significant challenges of palm oil is the deterioration/rancidity of this product with storage time [14]. Long-term storage of palm oil results in several biochemical reactions which influence its safety and sensory profiling. Hence, the need to prevent palm oil from rancidity became important. Nevertheless, post-contamination and inefficient preservation of edible oils after processing and preservation can further lead to palm oil spoilage [15].
Ginger (Zingiber officinale) is an important medicinal plant that belongs to the Zingiberaceae family. It is a native plant to South-Eastern Asia and is presently cultivated in many countries, such as Mexico, South Africa, Nigeria, Africa, China, Hawaii, Jamaica, etc. [16]. The medicinal value of biological products of oil extract from the ginger rhizomes is known and has been used as a food additive [17]. Interestingly, the use of ginger (Zingiber officinale) extract as a bio preservative agent of edible oils from spoilage microorganisms has been documented [18]. Essential oil from ginger possesses a broad-spectrum antimicrobial activity against food-associated pathogens [19]. The biopreservation potential of the ginger extract can help reduce the risk of food poisoning and improve organoleptic stability during food preservation [20]. Nevertheless, the use of the ginger extract in palm oil preservation is less documented. Therefore, this research was designed to assess the microbiological quality check and post-harvest effects of ginger extract on stored palm oil sourced from the Southern Senatorial District of Ondo State, Nigeria.
Materials and Methods
Sample source and treatment
Palm oil samples were collected from the open markets in five different towns in Irele local government area of Ondo state, namely, Ode-Irele, Ode-Omi, Ajagba, Akotogbo, and Iyansan. Ten samples, two from each market, were collected in triplicates. The samples were kept at ambient temperature and then transported to the laboratory aseptically for microbiological and physiochemical analyses (within 1-2 days after collection).
Palm fruits were picked from harvested bunches on the day of harvest (day 0) at Ode-Irele in Ondo State and then taken to the microbiology laboratory for analysis. After seven days, some palm fruits were also collected from the fermenting bunches and analyzed. Furthermore, several palm fruits were collected from the soft bunches before milling on the fourteenth day. After the palm fruit had been manually processed into palm oil, the palm oil sample was collected in a sterile container and sent to the laboratory for further analysis. Similarly, freshly processed palm oil was aseptically collected at a local oil mill in Okitipupa and then allowed to cool in open storage drums for 24 hours (day 0).
The oil was then divided into three groups in triplicate. For the first group, an equal volume of the oil was measured into McCartney bottles and then steam sterilized using a Gallenkamp autoclave at 121°C for 15, 20, and 25 minutes, respectively. For the second group, an equal volume of the oil was measured into six 350 Cl white transparent plastic bottles, in which three were set for ethanol ginger extract and the other three for water-ginger extract. The third group, which served as control, was kept in tightly sealed bottles without any treatment [21].
Source and preparation of plant materials
Fresh rhizomes of ginger were collected from local farms located at Okitipupa, Ondo State, Nigeria. The rhizomes were shade dried until all the water molecules evaporated, and the rhizomes were sufficiently dried for grinding. The periderm was removed, and the rhizomes were ground using an electrical laboratory blender into a fine powder and kept in an airtight container with proper labeling before analysis [22].
Preparation of plant extract and palm oil treatment
The plant extract was prepared using the method of [22] with minor changes. A total of 10 g of ginger powder was weighed into a 125 ml reagent container and the volume was completed to 100 ml of solvent at a ratio of 1:4 (powder/ethanol (0.1 M) and powder/water). The mixtures were set on an orbit machine for 72 hours with constant shaking. After that, a 0.45 m nylon membrane filter was used to filter the mixtures. The extracts were evaporated to dryness under reduced pressure at 40°C using a rotary evaporator.
Ginger extracts treatments
The method described by [22] was used with slight modification. Ethanol and water extracts of ginger at concentrations of 200 ppm (0.02 g per 100 ml oil), 400 ppm (0.04 g per 100 ml oil), and 600 ppm (0.06 g per 100 ml oil) were separately added to palm oil in white transparent plastic bottles of equal capacity. The treated oil samples were thoroughly shaken for proper mixing. Palm oil containing 200 ppm butylated hydroxytoluene (0.02 g per 100 ml oil) and that which contained no additive (control) was also set up. Each container was appropriately labeled and stored in an open place at room temperature ranging from 29°C to 35°C in the laboratory for 14 days. Each sample was collected and analyzed at a 7 days interval. Microbial succession patterns and palm oil degeneration (storability) were investigated.
Microbial analysis
The 10-fold serial dilution and pour plating procedures were used to isolate bacteria from processed palm oil, palm fruit, and raw treated palm oil. Nutrient agar (NA) and potato dextrose agar (PDA) were obtained from Sigma-Aldrich, Merck, Chemical Company, United States of America, and used as media. In a nutshell, a serial dilution was done by vortexing 1 ml of oil sample in 9 ml peptone water containing 10% v/v tween 20 solutions. 1 ml was pipetted from the stock solution and serially diluted up to 6 dilutions. Aliquots of the suspension (106) were carefully put into sterile Petri dishes and then plated with molten nutrient agar and potato dextrose agar that had already been sterilized. For bacteria, the plates were incubated at 28°C for 24 hours, while for fungi, the plates were incubated at 32°C for 72-96 hours. On the plate, microbes were selected for re-culturing based on their morphological appearance. After streaking on fresh microbiological media several times, pure cultures were produced. Cultural, biochemical, Gram staining, and sugar fermentation assays were used to identify the bacteria isolates. Catalase test, starch hydrolysis, citrate utilization test, Voges-Proskauer test, urease, oxidase test, and indole test are among the biochemical characteristics assessed. To evaluate a detailed analysis of fungal structure, fungal isolates were morphologically characterized using lactophenol blue stain under the microscope. Pure isolates were kept at 4°C until when needed.
Strict anaerobes
A loop of the test organism was injected into a sterile water-filled test tube, and 0.1 ml was pipetted into a sterile Petri plate. Nutrient agar was added and adequately stirred. After that, it was allowed to solidify before oil immersion to cut off the oxygen supply. The existence of growth demonstrated that the organism is a strict anaerobe [21].
Oxygen sensitivity test
Nutrient agar was sterilized and distributed into test tubes, which were then allowed to solidify vertically. The test organisms were inserted aseptically into each tube and cultured at 37oC for 5 days. The test organism’s movement from the top to the middle or bottom of the test tubes revealed whether the bacteria was aerobic, facultative, or anaerobic [23].
Physical and chemical analysis of palm oil
On day 0, week 1, and week 2, palm oil biochemical quality parameters, such as moisture content, free fatty acid (FFA), peroxide value, acid value, impurity level, and iodine level, were examined [12].
Moisture content
The oven-dry method was used to determine the palm fruit samples. After weighing the evaporation dish, 5 g of each palm fruit was added and weighed again. For 3 hours, the dish containing the samples was maintained in a 105°C oven (until a constant weight was attained). After drying, the palm fruit was weighed again after cooling in a desiccator.
% moisture content was calculated as:
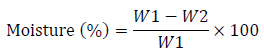
Where W1 = weight of dish + weight of palm fruit before drying (g)
W2 = weight of dish + weight of palm fruit after drying (g)
Determination of free fatty acid value
Five milliliters of palm oil were dispensed and weighed into a sterile 250-milliliter conical flask. To completely release the fatty acid, an aliquot of ethanol (50 ml) was added to the oil and cooked on a hot plate with steady swirling. The indicator used in the titration was phenolphthalein. The ethanol-oil mixture was titrated against 0.1 N NaOH until it reached a reasonably pink endpoint [24]. Using the formula below, the free fatty acid content of palm oil was computed and recorded.
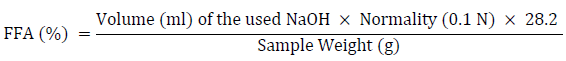
The ethanol-oil samples’ acid values were calculated by multiplying the free acid value by two.
Determination of peroxide value
The peroxide value was calculated by titrating the oil’s potassium iodide solution with an aqueous sodium thiosulphate solution using starch as an indicator. For this approach, five grams of oil was weighed into a 250-ml conical flask. In a 3:2 ratio, a mixture of glacial acetic acid and trichloromethane chloroform was added. A 0.5 ml saturated potassium iodide solution (144 g per 100 g distilled water) was also added. Following the shaking, 30 ml of water was added. The solution was titrated with 0.1 M sodium thiosulphate until the yellow tint was nearly undetectable. The starch indicator was added to the titration process at a rate of 0.5 mL per minute while shaking vigorously until the blue-black color vanished. The same approach was used to examine a blank sample that did not contain any palm oil samples. The differences between the two sodium thiosulphate titer levels used were calculated as follows:
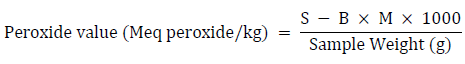
Where: S = Sample titer (mL), B = Blank titer (mL), M = Molarity of thiosulphate
Impurity level
The impurity level of palm oil was determined using the method of [25].10 ml of palm oil was weighed into a beaker with extra hexane (20 – 30 ml), boiled to 105°C, and filtered. Hexane was used to clean the residue on the filter paper. After allowing the filtered material to cool in a desiccator, the residual weight of particles on the filter paper was estimated as follows:
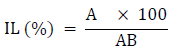
Where: IL is Impurity Level (%), A = weight of desiccated residual particles (g), AB = total weight of the sample before treatment (g)
Iodine level
The iodine value (IV) was determined using the Wijs solution method [24].
Statistical analysis
Statistical analysis was carried out using one-way analysis of variance (ANOVA) with Duncan’s Multiple Range Test (DMRT) as the Post-Hoc Test. Statistical Package for Social Sciences Software (SPSS) and data was expressed as mean±standard deviation. Differences were considered statistically significant at p < 0.05.
Results
Microbial analysis of palm fruit, processed palm oil, and treated raw palm oil
At days 7 and 14, high bacterial counts of 6.8 x 105 CFU/ml and fungal counts of 8.5 x 103 CFU/ml were recorded from the stored palm fruits isolates after 7 and 14 days, respectively (Table 1). Also, a high bacterial count of 3.5 x 105 CFU/ml and 3.7 x 105 CFU/ml and fungal count of 4.5 x 103 CFU/ml and 5.3 x 103 CFU/ml were recorded from the palm oil samples collected from the Ode-Omi market. The microbial count in the treated raw samples was lower compared to the control with the higher values of 7.5 x 105 CFU/ml and 8.0 x 105 CFU/ml for bacteria and 1.8 x 103 CFU/ml and 2.0 x 103 CFU/ml for fungi at days 7 and 14, respectively. There was no microbial growth on the cultured media inoculated with sterilized samples (Table 2).
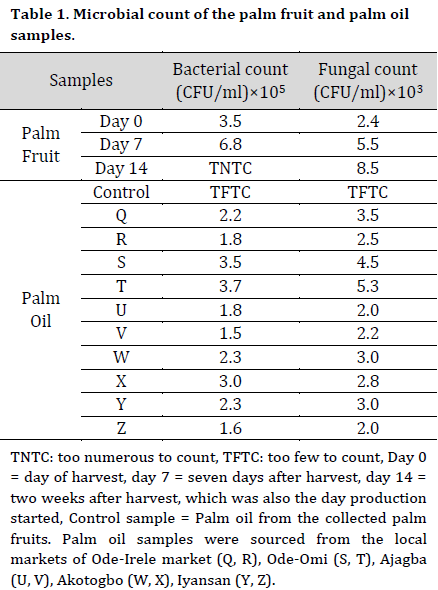
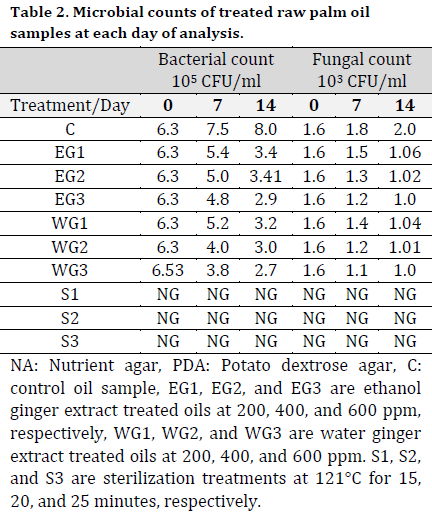
Morphological and biochemical characterization of the microbial isolates
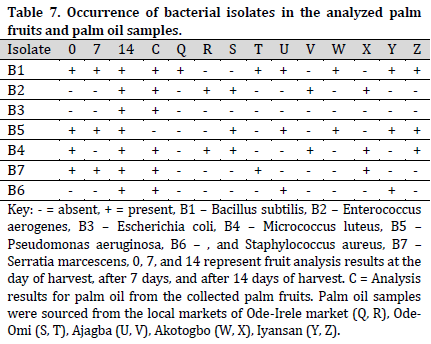
The bacterial species identified from palm fruit and palm oil included Bacillus subtilis, Enterococcus aerogenes, Escherichia coli, Micrococcus luteus, Pseudomonas aeruginosa, Staphylococcus aureus, Serratia marcescens (Table 3), while in the treated raw palm oil, B. subtilis, E. aerogenes, M. varians, P. aeruginosa, and S. saprophyticus were isolated (Table 4).
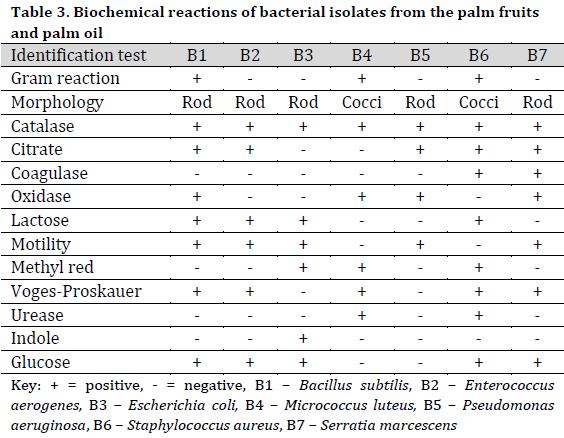
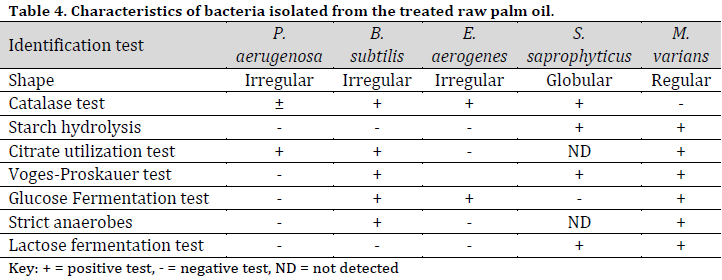
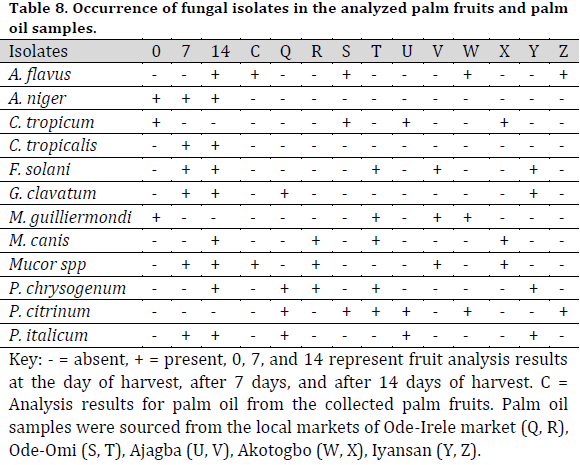
The identifiable fungal isolates from palm fruits and processed palm oil included Aspergillus flavus, A. niger, Chrysosporium tropicum, Fusarium solani, Geotrichum clavatum, Meyerozyma guilliermondii, Microsporum canis, Mucor spp., Penicillium chryogenum, P. citricum, and P. italicum (Table 5). Similarly, A. niger, M. guilliermondii, P. citricum, and Cochliobolus spp were isolated from the treated raw palm oil (Table 6).

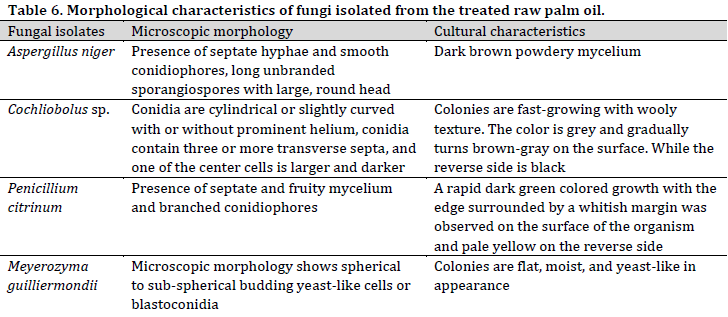
Microbial occurrence in palm oil samples
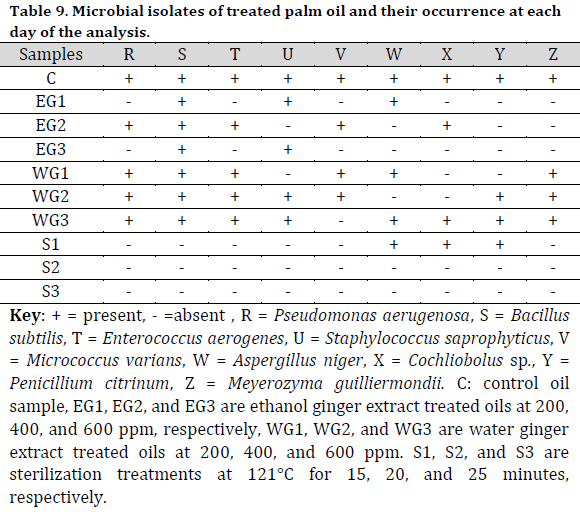
Bacillus subtilis and P. aeruginosa were found in the treated raw palm oil collected at days 0, 7, and 14; as well as processed palm oil samples obtained from the Ajagba, Akotogbo, and Iyansan markets. Except for P. aeruginosa, all of the microorganisms were detected in the control samples. In addition, only B. subtilis showed to be prominent in processed palm oil from Ode-Irele (Q) (Table 7). When compared to the control and other samples, the dominance of A. flavus, A. niger, C. tropicalis, F. solani, G. clavatum, M. canis, Mucorspp, P. chrysogenum, and P. italicum was profound in the treated raw palm oil samples stored at day 14 (Table 8). The occurrence of microorganisms in the treated raw palm oil on each day of the analysis is shown in Table 9.
Physical and chemical/quality analysis of palm oil samples
According to the Standard Organization of Nigeria (SON), the free fatty acid concentration of the processed palm oil samples was greater than the control and acceptable level (Fig. 1). The peroxide and impurity content readings were lower than the allowed limit, with greater values as compared to the control. All of the processed palm oil samples had low moisture content compared to the control and the allowed limit.
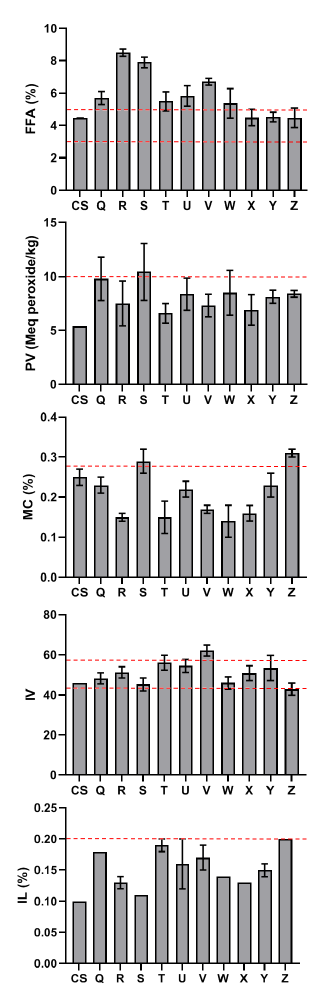
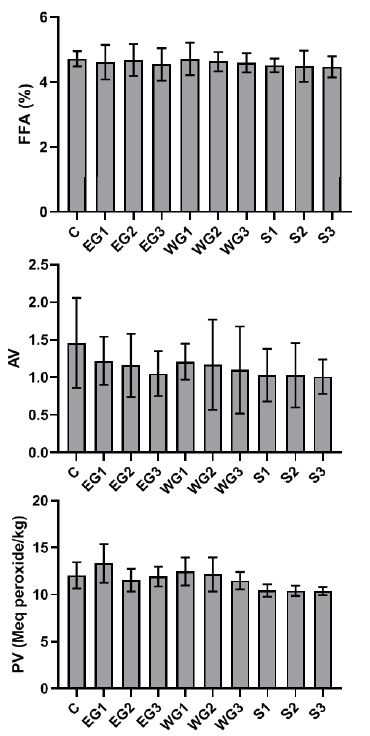
The iodine concentration of the processed palm oil samples was higher than the control, and the SON allowed limit, except for the processed palm oil gathered from the Iyansan market (Z). The chemical quality of raw palm oil treated with ginger extract and sterilization revealed that the control sample had greater free fatty acid and acid value than the palm oil samples, whereas ethanol ginger extract had a high peroxide value of 13.33 at 200 ppm (Fig. 2).
Discussion
The microflora of edible oil is a reflection of the oil’s quality, as well as the environmental and hygienic conditions of the equipment used in its production, packing, and handling [26]. Maintaining hygienic parameters and quality control during food processing is critical for ensuring a safe supply of food commodities on the market [27]. The bacterial load of the control palm oil sample obtained from the mill is below the Nigerian Agency for Food and Drug Administration’s (NAFDAC) minimum acceptable microbiological level, which stipulates that the maximum allowable number of organisms in a sample unit of oil should not exceed 2, with an acceptable microbiological level of 104 CFU/ml. There was no microbial development in the sterilized oil samples. Furthermore, the bacterial and fungal load in palm oil containing ginger extracts was substantially lower than the control setup. This observation meant that the microorganisms were killed during the sterilization process. The bacterial load in palm oil with ginger extracts was within the acceptable range of microorganisms (104 CFU/ml). The majority of the microorganisms found in palm fruits were unable to withstand the palm oil extraction process. These bacteria could have entered the palm oil as a result of processing and handling. Food contamination caused by poor sanitation, the use of dirty water, and contamination by food processors and handlers have also been reported to impact the nutritional quality and acceptance of food products [28][29]. The low microbial counts observed in the fresh oil samples are in accordance with [30], who reported low microbial counts in raw palm oil while investigating chemical characteristics of deteriorated palm oil. Also, the high microbial counts found in oil samples obtained from the market-places can be due to the oil exposure to the air in the market, prompting spore-forming microbes to proliferate. Enterobacter aerogenes, for instance, can be found in the environment and water [31]. Thus, their presence in palm oil samples might indicate contamination from the surrounding environment.
Individuals who consume oil contaminated with harmful microorganisms may develop gastroenteritis and other gastrointestinal illnesses. Staphylococcus aureus may produce enterotoxin and has been known to persist in hostile conditions for lengthy periods [32]. Their presence in
palm oil revealed that the oil was handled in an unsanitary manner by the processors [33]. The ability of A. niger and Mucor spp to thrive in oil by producing enzyme lipase has been reported [34]. Because of their ability to produce spores, these fungi have survived the heat and the anaerobic condition of the oil. A. flavus capability to produce aflatoxin rendered it a public health concern [35]. These microorganisms represent a looming danger to public health, which emphasizes the importance of sanitizing conditions during palm oil production.
The mean values of several selected quality parameters of palm oil preserved for one month with various concentrations of ethanol and water ginger extract and sterilized palm oil were evaluated. Free fatty acid, acid value, and peroxide value were selected as reference indicators of hydrolytic and oxidative rancidity of edible oils [36]. The increased values of the aforementioned parameters significantly diminish the nutritional value of edible oils [37]. The iodine value in palm oil and other vegetable oils is used to detect adulteration [35]. The iodine value found agrees with [30], who reported that the higher the degree of unsaturated fats (i.e., the higher the iodine value), the more easily the oil or fat gets rancid. It has been reported that the comparative analysis of processed oil can be employed to determine the iodine level in oils [38]. The high iodine value found in this study may account for the rancidity in the examined oil samples. The degree of oxidation in oil and the level of deterioration of oil and fats can be determined by the peroxide value [35][39]. Although PV of the palm oil samples obtained in this study increased drastically during storage, no signs of rancidity were observed in control or treated oils, which agrees with an experimental result claiming that rancidity commonly appears when peroxide values are between 20 and 40 mEq/kg [35]. Rancidity is a sign that fats and oils are deteriorating. Due to the used production processes and storage conditions, the PV of the oil samples showed that the oils sold within the South Senatorial District of Ondo State might have turned rancid.
The maximum allowable amount for free fatty acid, according to SON, is 5% [40]. The results of FFA obtained from this study were similar to the findings of Ekwenye [30] who reported higher FFA values in oil samples exposed to normal room conditions (room temperature 28oC) compared to the corresponding unexposed samples with low FFA values. It could also be related to the accompanying fungi’s degradation of glycerides and the palm oil’s rapid exposure to heat and sunlight. [35] previously observed that the glycerides in the oil can be degraded by lipase or other enzymes, and this decomposition can be expedited by heat/sunlight. Palm oil’s quality is harmed by high levels of FFA, which can cause a variety of health and environmental problems [41]. Impurities are said to enter into crude palm oil during the final extraction and purification steps, as reported by [42]. Handling and marketing processes are also thought to have introduced these contaminants to unrefined palm oil. When compared to the SON acceptable limit, the palm oil studied in this study had a moderate quantity of contaminants. Water ginger extract in palm oil had a lower value than ethanol ginger extract in palm oil. In terms of controlling microbial proliferation in palm oil samples that might cause hydrolytic rancidity of palm oil under storage, sterilization outperforms ginger extract treatment based on the microbial loads recorded from this study. The peroxide value in all sterilized palm oil samples was the lowest. As a result, sterilization is more efficient in preventing palm oil’s oxidative rancidity.
Conclusion
Traditional oil extraction processes are sometimes associated with poor hygiene, resulting in low-quality oil compared to oil produced by commercial oil mills. The major palm oil processing methods employed in Irele local government area of Ondo State’s in the Southern Senatorial District are traditional or semi-mechanized. Certain palm oil samples received from the market did not fulfill the SON criteria for palm oil. The microbiological and physicochemical features of the oil samples from the markets in this area, such as peroxide value, free fatty acid, and iodine level, all exceeded the limits, indicating improper processing and handling of palm oil by extractors and vendors. The high peroxide value and free fatty acid content suggest that some palm oil samples have deteriorated. Despite their low microbial burden, several of the bacteria detected can cause health problems in people who ingest the food without heat processing. Both hydrolytic and oxidative rancidity, as well as the bacteria that cause palm oil deterioration, were efficiently prevented by sterilization, in addition to water and ethanol extracts of ginger. Since sterilization retains the flavor and taste, it is more successful in fighting hydrolytic rancidity. As a result, sterilizing and ginger extracts can be employed to keep palm oil for an extended period while maintaining its physiochemical properties.
Acknowledgments
The authors acknowledge the efforts of staff members in the Department of Biological Sciences of the Olusegun Agagu University of Science and Technology, Okitipupa, Nigeria, during this research. The research was carried out without funding.
References
| 1 | Pedapati A, Tyagi V, Yadav S, Brahmi P, Murugesan P. Present status and future priorities for the introduction of oil palm in India. Int. Biannual J. Environ. Sci. 2013;3:134-44. |
| 2 | Murugesan P, Somasundaram G, Pandey V. Evaluation of seedlings of oil palm germplasm from Sierra Leone and Senegal in India. Indian J. Hortic.2021;78:61-6. DOI |
| 3 | Ghodsi R, Nosrati R. Effects of minor compounds of edible oils on human health. Curr. Nutr. Food Sci. 2020;16:1196-208. DOI |
| 4 | Albuquerque TG, Costa HS, Silva MA, Oliveira MB. Are chloropropanols and glycidyl fatty acid esters a matter of concern in palm oil?. Trends Food Sci Technol. 2020;105:494-514. DOI |
| 5 | Adeleke BS, Babalola OO. Oilseed crop sunflower (Helianthus annuus) as a source of food: Nutritional and health benefits. Food Sci. Nutr. 2020;8(9):4666-84. DOI |
| 6 | Dian N, Hamid R, Kanagaratnam S, Isa WA, Hassim NAM, Ismail NH, Omar Z, Sahri MM. Palm oil and palm kernel oil: Versatile ingredients for food applications. J. Oil Palm Res.2017;29:487-511. DOI |
| 7 | Tan JP, Jahim JM, Harun S, Wu TY. Overview of the potential of bio-succinic acid production from oil palm fronds. J. Physical Sci. 2017;28:53-72. DOI |
| 8 | Purnama KO, Setyaningsih D, Hambali E, Taniwiryono D. Processing, characteristics, and potential application of red palm oil-A review. Int. J. Oil Palm.2020;3:40-55. DOI |
| 9 | Suryani S, Sariani S, Earnestly F, Marganof M, Rahmawati R, Sevindrajuta S, Mahlia TMI, Fudholi A. A comparative study of virgin coconut oil, coconut oil and palm oil in terms of their active ingredients. Processes.2020;8:402. DOI |
| 10 | Woodfield HK, Harwood JL. Oilseed Crops: linseed, rapeseed, soybean, and sunflower. In Encyclopedia of Applied Plant Sciences (Second Edition), Thomas, B., Murray, B.G., Murphy, D.J., Eds. Academic Press: Oxford, 20173;4-38. DOI |
| 11 | Aminu FO, Umoh J. Factors influencing economic performance of palm oil producers in Akwa Ibom State, Nigeria. Int. J. Oil Palm.2020;3:69-77. DOI |
| 12 | Chinedu EE, Ebere EC, Emeka AC. Quality assessment of palm oil from different palm oil local factories in Imo State, Nigeria. World Sci. News. 2017;88:152-67. |
| 13 | Nwaiwu O, Itumoh M. Chemical contaminants associated with palm wine from Nigeria are potential food safety hazards. Beverages.2017;3:16. DOI |
| 14 | Chuku E, Chuku O. Fungi associated with the rancidity of palm oil. Niger. J. Mycol. 2016;8:122-30. |
| 15 | Rawat S. Food spoilage: Microorganisms and their prevention. Asian J. Plant Sci. Res.2015;5:47-56. |
| 16 | Olaniran AF, Abiose SH, Adeniran AH. Biopreservative effect of ginger (Zingiber officinale) and garlic powder (Allium sativum) on tomato paste. J. Food Safety.2015;35:440-52. DOI |
| 17 | Al-Dhahli AS, Al-Hassani FA, Alarjani KM, Yehia HM., Al Lawati WM, Azmi SNH, Khan SA. Essential oil from the rhizomes of the Saudi and Chinese Zingiber officinale cultivars: Comparison of chemical composition, antibacterial and molecular docking studies. J. King Saud University-Sci.2020;32:3343-50. DOI |
| 18 | Ahmed LI, Ibrahim N, Abdel-Salam AB, Fahim KM. Potential application of ginger, clove and thyme essential oils to improve soft cheese microbial safety and sensory characteristics. Food Biosci.2021;42:101177. DOI |
| 19 | Jiménez-Reyes MF, Carrasco H, Olea AF, Silva-Moreno E. Natural compounds: A sustainable alternative to the phytopathogens control. J. Chil. Chem. Soc. 2019;64:4459-65. DOI |
| 20 | El-Zawahry A, El-Wahed A. Effect of ginger rhizomes extracts on keeping quality and oxidative stability of UF-white soft cheese. J. Food Dairy Sci.2018;9:333-338. DOI |
| 21 | Okogbenin O, Okogbenin E, Okunwaye T, Odigie E, Ojieabu A. Isolation of food pathogens from freshly milled palm oil and the effect of sterilization on oil quality parameters. J. Food Secur. 2014;2:65-71. |
| 22 | Arawande J, Ijitona O, Olatide M. Effectiveness of adding ginger extract in preserving crude peanut oil. J. Food Technol. Preservation.2018;2:2-12. |
| 23 | Monica C. District Laboratory Practice in Tropical Countries. Second Editon, Cambridge University Press, Cambridge, UK. 2006. |
| 24 | Ohimain EI, Izah SC, Fawari AD. Quality assessment of crude palm oil produced by semi-mechanized processor in Bayelsa state, Nigeria. Discourse J. Agric. Food Sci. 2013;1:34-46. |
| 25 | Albert MME, Laverdure DEE, Paul K. Assessment of the quality of crude palm oil from smallholders in Cameroon. J. Stored Prod. Postharvest Res.2011;2:52-8. |
| 26 | Ebrahimian E, Seyyedi SM, Bybordi A, Damalas CA. Seed yield and oil quality of sunflower, safflower, and sesame under different levels of irrigation water availability. Agric. Water Manag. 2019;218:149-157. DOI |
| 27 | Faille C, Cunault C, Dubois T, Benezech T. Hygienic design of food processing lines to mitigate the risk of bacterial food contamination with respect to environmental concerns. Innov. Food Sci. Emerg. Technol.2018;46:65-73. DOI |
| 28 | Abdullahi BS, Maiha SB, Lawal HK. Hygiene, food safety practices and sanitation in some food service centres in Zaria, Kaduna State, Nigeria. Amer. J. Food Sci. Technol.2020;8:206-10. |
| 29 | Nkere CK, Ibe NI, Iroegbu CU. Bacteriological quality of foods and water sold by vendors and in restaurants in Nsukka, Enugu State, Nigeria: A comparative study of three microbiological methods. J. Health Popul. Nutr.2011;29:560-6. DOI |
| 30 | Ekwenye UN. Chemical characteristics of palm oil biodeterioration. Biokemistri.2006;18:141-9. |
| 31 | Izah S, Ohimain E. Microbiological quality of palm oil used in Nigeria: Health impacts perspective. Point J. Bot. Microbiol. Res.2016;46. |
| 32 | Al-Bahry S, Mahmoud I, Al-Musharafi S, Sivakumar N. Staphylococcus aureus contamination during food preparation, processing and handling. Int. J. Chem. Engin. Applications.2014;5:388. DOI |
| 33 | Baş M, Ersun AŞ, Kıvanç G. The evaluation of food hygiene knowledge, attitudes, and practices of food handlers’ in food businesses in Turkey. Food Control. 2006;17:317-22. DOI |
| 34 | Agu K, Ogbue M, Abuchi H, Onunkwo A, Chidi-Onuorah L, Awah N. Lipase production by fungal isolates from palm oil-contaminated soil in Awka Anambra State, Nigeria. Int. J. Agric. Biosci.2013;2:386-90. |
| 35 | Ezediokpu M, Okerentugba P, Omorodion N, Ibienebo T. Quality assessment of unbranded palm oil distributed in five local markets in port Harcourt, Rivers state Nigeria. IOSR J. Agric. Vet. Sci.2015;8:27-32. |
| 36 | Okparanta S, Daminabo V, Solomon L. Assessment of rancidity and other physicochemical properties of edible oils (mustard and corn oils) stored at room temperature. J. Food Nutr. Sci.2018;6:70-5. DOI |
| 37 | Simon KP, Aruna M, Mandha J, Rao D. Nutritional evaluation and oil blending studies of different oils. Int. J. Food Sci. Nutr.2017;2:101-5. |
| 38 | Birnin-Yauri U, Garba S. Comparative Studies on some physicochemical properties of baobab, vegetable, peanut and palm oils. Niger. J. Basic Appl. Sci.2011;19:64-7. DOI |
| 39 | Zahir E, Saeed R, Hameed MA, Yousuf A. Study of physicochemical properties of edible oil and evaluation of frying oil quality by Fourier Transform-Infrared (FT-IR) Spectroscopy. Arab. J. Chem.2017;10:3870-6. DOI |
| 40 | Aphiar AE, Ikechukwu RN. Quality assessment of physicochemical properties of palm oil from different palm oil mills in Isoko, Delta State. Int. J. Innov. Res. Adv. Stud..2019;6:31-5. |
| 41 | Torri L, Bondioli P, Folegatti L, Rovellini P, Piochi M, Morini G. Development of Perilla seed oil and extra virgin olive oil blends for nutritional, oxidative stability and consumer acceptance improvements. Food Chem.2019;286:584-91. DOI |
| 42 | Orji MU, Mbata TI. Effect of extraction methods on the quality and spoilage of Nigerian palm oil. Afr. J. Biochem. Res.2008;2:192-6. |
Cite this article:
Oguntimehin, M., Adeleke, B. Microbiological, physical, and chemical assessment of palm oil under ginger extracts and sterilization treatment. DYSONA – Applied Science, 2022;3(2): 33-45. doi: 10.30493/das.2022.313488
