Francesco Zagami 12*; Michael Alabboud 3
1, Tabaka Mission Hospital, Tabaka, Kisii, Kenia
2, Medicitalia Srl, Foro Bonaparte, Milano, Italy
3, Department of Horticulture, Al-Baath University, Homs, Syria
E-mail:
francesco_zagami@virgilio.it
Received: 15/08/2023
Acceptance: 20/03/2023
Available Online: 21/09/2023
Published: 01/10/2023

Manuscript link
http://dx.doi.org/10.30493/DLS.2023.789777
Abstract
Myasthenia gravis (MG) is an autoimmune disease known to affect the transmission of signals at the neuromuscular junction. Despite the utilization of multiple methods to alleviate symptoms and improve the quality of life for individuals with MG, a comprehensive and entirely effective regimen has yet to be achieved. Pyridostigmine bromide and huperzine A are pharmacological agents that function as inhibitors of red blood cell-acetylcholinesterase. These compounds are extensively employed in the treatment protocols for myasthenia gravis. Nevertheless, the distinct disparities between huperzine A and Pyridostigmine bromide necessitate further investigation into the potential of these drugs in relieving the clinical symptoms of MG. The current research investigated MG symptom enhancement after four weeks of huperzine A, pyridostigmine bromide, and an immunomodulatory integrated regimen. Six MG patients were monitored for subjective enhancements in MG symptoms and quality of life as well as red blood cell-acetylcholinesterase activity and acetylcholine receptor antibody binding reduction before and four weeks after the initiation of the treatment protocol. The results showed that MG symptoms were reduced in all the monitored cases with an average overall enhancement of 80.6±5.5%. Additionally, the quality of life questionnaire revealed an overall enhancement of 72±5.7%. Additionally, the red blood cell-acetylcholinesterase activity was regulated in all patients within the pre-set therapeutic target of 25-35 U/g Hb. Although a decent reduction in acetylcholine receptor antibody binding was achieved in all patients, none of them reached normal levels for this index. The present findings on the integration of huperzine A and other immunomodulatory drugs into the therapy regimen for MG are exceedingly promising, particularly in terms of the potential reduction in dosage requirements or even the elimination of pyridostigmine bromide administration. However, it is imperative to examine different therapeutic approaches in future research endeavors.
Keywords: Myasthenia gravis, Huperzine A, Pyridostigmine bromide, RBC-AChE assay
Introduction
Myasthenia gravis (MG) is a chronic autoimmune neuromuscular disorder characterized by the weakening of skeletal muscles. The primary underlying mechanism of this condition is the impairment of the postsynaptic component at the neuromuscular junction (NMJ), primarily resulting in the diminished functionality of nicotine acetylcholine receptors (nAChRs) on the muscle membrane [1]. These receptors consist of five homologous subunits that are arranged circularly around a central pore. These receptors can be classified into two groups with the first primarily present in the skeletal muscles of vertebrates and playing a role in neuromuscular transmission at the neuromuscular junction (NMJ), and the second is the neuronal type, which is predominantly found in both the peripheral nervous system (PNS) and central nervous system (CNS). Additionally, the neuronal type of nAChRs can also be found in non-neuronal tissues such as lymphocytes, platelets, macrophages, myelo- and erythrocyte progenitors, as well as mature erythrocytes [2][3].
Although the nAChR exhibits a high level of immunogenicity, with its antibodies being the primary causative agents of myasthenia gravis, it is noteworthy that this autoimmune condition appears to be relatively uncommon based on statistical data [4]. This assumption may be associated with the additional neurotransmitter activity of ACh, which has the ability to reduce the release of proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukins (IL-1 and IL-6). This mechanism plays a crucial role in the inhibition of cytokine release through a pathway known as the “cholinergic anti-inflammatory pathway” [5].
Immune cells have the capability to express many elements of the cholinergic system, including choline acetyltransferase (ChAT), which is responsible for the synthesis of ACh, acetylcholinesterase, which is involved in the degradation of ACh, and nAChR. Non-neuronal ACh exerts its effects on nAChR through autocrine and paracrine mechanisms, hence modulating immune responses [6]. The cholinergic system plays a crucial role in delicately regulating the vast immune system limiting the pathological damage caused by over-inflammation in the context of autoimmune and inflammatory diseases. The mechanism involves the inhibition of acetylcholine receptors on macrophages, leading to a decrease in the production of chemokines CCL-2/7. Consequently, this results in a reduction in the recruitment of inflammatory macrophages [7]. Moreover, there is a transition in the functional phenotype of macrophages from M1 to M2. The equilibrium between Th17 and Treg differentiation is altered in favor of Tregs in order to mitigate inflammation. However, T helper cell subsets have a tendency to undergo Th1 polarization. The activation of cholinergic receptors has been observed to exert additional influence on the levels of cytokines and chemokines via intracellular signaling pathways (Fig. 1) [7].
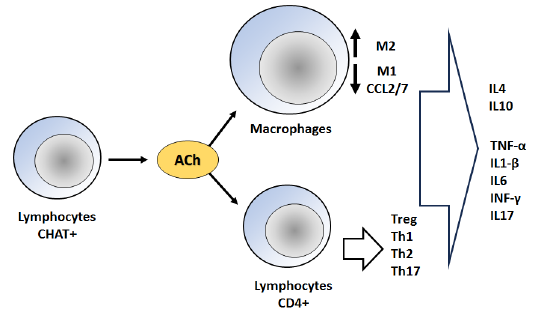
The released ACh binds to the postsynaptic nicotinic/muscarinic receptors (M1) located in the muscle and immune system. This binding leads to the generation of endplate potential (EPP) at the neuromuscular junction (NMJ), resulting in muscle contraction. Additionally, ACh binding to the immune system receptors induces anti-inflammatory activity. These actions are regulated by the enzyme AChE, which breaks down ACh into acetic acid and choline. The resulting products are then reabsorbed into the presynaptic membrane or taken up by immune cells to be reformed into ACh through the action of ChAT [8].
ACh binds to the M1 receptors found on erythrocytes, resulting in the modulation of red blood cell rheology. This modulation is characterized by a decrease in aggregation and an increase in deformability of the cells. These effects are achieved through alterations in membrane lipid fluidity. The purpose of this modulation is to facilitate the regulation of tissue oxygenation and blood distribution across different vascular territories. Additionally, it has been observed that the AChE membrane enzyme has a role in modulating plasma ion concentrations and nitric oxide metabolites. This modulation occurs through intracellular nitric oxide translocation and metabolism, as well as band 3 protein phosphorylation [9][10].
Cholinesterases, specifically AChE, are carboxylesterases that exhibit a high degree of polymorphism. They possess a wide range of substrate specificity, leading to their classification as either red blood cell-acetylcholinesterase (RBC-AChE) or butyrylcholinesterase (BChE). RBC-AChE is found in erythrocytes, other blood cells, cholinergic synapses at neuromuscular junctions, as well as in central nervous system (CNS) connections, inter-neuronal connections of the peripheral nervous system, and neuroglandular and neuromuscular junctions of the parasympathetic nervous system. On the other hand, BChE is classified based on its substrate specificity and sensitivity to specific inhibitors [11].
The activity of RBC-AChE is regulated by the phosphorylation of the membrane band 3 protein. This phosphorylation enables the activation of pathways that control the transport of nitric oxide (NO) to tissues under conditions of low partial pressure of oxygen (PaO2), while also facilitating its elimination under conditions of high PaO2. The erythrocytes’ capacity to transport and retain NO is contingent upon the integrity of their membrane, the activation status of AChE, and the specific molecular protein conformations it assumes [12].
AChE serves as a biomarker for inflammation in various diseases, including cancer. In fact, AChE levels in humans are subject to fluctuations due to interindividual variability in ethnic and genetic traits, as well as other factors such as age, gender, reproductive and health status, drug use, medications, and the presence of specific diseases. The detection of elevated activity of RBC-AChE may suggest the initial stages of sterile inflammation, serving as an indicator of cholinergic downregulation that supports the proinflammatory phase of the early immune response. Conversely, BChE exhibits a contrasting pattern, displaying a significant decrease in activity following sterile inflammation [13].
In the context of myasthenia gravis (MG), a significant autoimmune disorder affecting the NMJ, the primary focus is on the nAChR. The presence of autoantibodies against this receptor initiates a series of events, beginning with receptor internalization, followed by complement-mediated lysis of the postsynaptic membrane. Ultimately, these processes lead to the impairment of muscle contraction. The manifestation of muscular symptoms resulting from impaired signal transmission is contingent upon the death of receptors, and these symptoms can be alleviated through the restoration of the receptor pool via the process of new gene expression [14].
Approximately 80% of patients diagnosed with MG, namely those with nAChR-Ab, exhibit the presence of muscle nAChR antibodies, which have been identified as potentially harmful. Approximately 10-15% of the remaining patients diagnosed with MG exhibit antibodies targeting MuSK, LRP4, or agrin, which are all crucial NMJ components involved in the functioning of nAChRs. Conversely, a subset of approximately 5-10% of patients are categorized as “seronegative” [4].
The treatment strategy for MG has historically involved the administration of AChEI to augment the concentration of ACh in the synaptic cleft. This approach aims to induce a heightened level of the neurotransmitter, resulting in sustained depolarization of the postsynaptic nerve and subsequent uncontrolled firing of the synapse. Consequently, the targeted muscle remains in a contracted state. This therapeutic approach has been supported by the established understanding of MG’s pathogenesis since 1934. Currently, the administration of AChEIs is commonly combined with corticosteroids (CS), intravenous (IV) or subcutaneous (SC) immunoglobulin (Ig), plasmapheresis (PLEx), immunosuppressive agents (IS), complement inhibitors (CI), or biologic therapy [15].
Previous research has demonstrated that the absence of AChEI and/or CS leads to increased rates of exacerbation. As a result, the combined use of these medications is considered a primary treatment approach for MG. However, it is important to note that the use of these medications can often result in adverse reactions or complications, which can negatively impact the quality of life for patients. Nevertheless, despite these challenges, the treatment effectively reduced mortality rates [16].
Various agents are used in the systematic treatment of MG [17]. In this context, Huperzine A (HupA), a natural compound frequently employed in Eastern medicine, exhibits similar qualities to other naturally occurring compounds with appealing properties. This compound is derived from Huperzia serrata plant, which is currently marketed as a nutraceutical in the United States. This particular extract has demonstrated notable efficacy as a reversible inhibitor of cholinesterase, exhibiting a high degree of selectivity towards specific acetylcholinesterase enzymes [18]. Previously, an ample evidence of the specificity of HupA inhibition on AChE was provided [19]. This observation demonstrates that HupA exhibits selectivity as an RBC-AChE inhibitor, in contrast to non-selective inhibitors such as pyridostigmine bromide (PYR-B), which have a pronounced impact on BChE [20].
The presence of HupA resulted in an increase in the amplitude of muscle contraction through indirect electrical nerve stimulation in an in vitro setting. HupA demonstrates the ability to traverse the blood-brain barrier; nevertheless, the quantifiable radioactivity associated with the radiolabeled medication is shown to be limited in each organ assessed during a 24-hour timeframe. After a period of seven days following the administration of a solitary dose, it was observed that 86.1% of the drug was excreted through the urine, with 84.9% of the expelled drug being eliminated within the initial 24-hour timeframe. Additionally, 5.5% of the drug was eliminated through the digestive tract [21]. The various differences between HupA and PYR-B [20-23] prompt the exploration of the potential of both compounds in enhancing MG clinical symptoms in the current research while concurrently minimizing pharmacological dosage. The levels of anti-AChE antibodies and RBC-AChE activity were assessed as a reflection of synaptic AChE.
Material and Methods
Participants
A total of six homecare patients living in Sicily (Italy), consisting of four females and two males, were included in the study. The average age of the patients was 44.5 years, with a standard deviation of 14.85 years. These patients were accurately diagnosed in several Italian Neurological Department hospitals for myasthenia gravis (MG) based on typical clinical symptoms and positive results for AChR antibody (AChR-Ab) binding, in accordance with the MG Foundation of American clinical classification [24]. The patients were initially treated with PYR-B, but experienced various short-term side effects or difficulties in achieving optimal dosage, leading to dose reductions or discontinuation of the treatment.
Treatment program
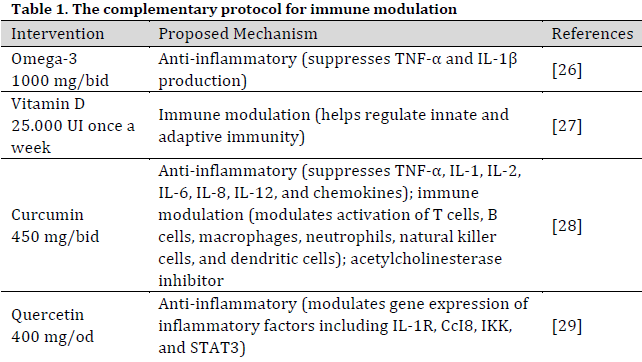
The patients in the study received HupA (Livitté® 100 mcg tablet) as an alternative or supplement to PYR-B therapy (Mestinon® 60 mg tablet), along with a standardized complementary protocol (Table 1). The dosage and timing of administration were adjusted based on the patient’s clinical condition and laboratory findings, as detailed in the subsequent results section. The integration of complementary medicine with conventional treatments has become increasingly prevalent. This approach emphasizes the importance of managing nutrition, stress, lifestyle, and physical exercise in order to facilitate the recovery process and enhance quality of life. Additionally, this approach offers the potential to mitigate the adverse effects associated with pharmaceutical interventions. The patients voluntarily requested to be treated at Medicitalia diagnostic research office. Thus, the informed consent form was signed by all participants, and the preservation of participant anonymity was ensured [29].
Subjective monitoring
A daily self-evaluation was conducted by answering a Local Patient Identifier (LPI) questionnaire (Supplementary Table 1), with a daily recording of the intensity of the disease-specific and non-specific symptoms to facilitate the identification of the curative and/or collateral effects. This self-evaluation was conducted before and 4 weeks after treatment program initiation.
The score assigned for each symptom ranged from 0 to 5 based on the patient’s subjective perception. This approach aims to enhance the objectivity of disease assessment by establishing a correlation between symptom severity and drug administration. Participants were instructed to use a numerical scale ranging from 0 to 5 to indicate the severity of symptoms to describe the absence, mild presence, moderate presence, moderate severe presence, serious presence, and very serious presence of the symptoms, respectively. Subsequently, a comprehensive assessment was conducted to examine the perception of treatment outcomes. Patients were administered the MG-QoL15 [30], a 15-item questionnaire (Supplementary Table 2), which aimed to gauge their perception of quality of life (QoL) during the four weeks preceding the treatment as well as the four weeks following it. This questionnaire encompasses the physical, social, and psychological aspects. Despite the recommendations of several studies to utilize the 3-response option (ranging from 0 to 2) for this particular questionnaire [31][32], a decision was made to adopt a grading system similar to that of the LPI questionnaire. This choice was motivated by the intention to minimize potential confusion among patients.
The data obtained from the questionnaires (self-evaluation and quality of life) before and after treatment were used to calculate the improvement percentage of each case:
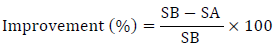
where
SA: Total questionnaire score for the subject after 4 weeks of the proposed therapy
SB: Total questionnaire score before initiating the proposed therapy
In the context of data interpretation, a predetermined criterion was established to classify patients based on percentage values. Specifically, if the percentage value exceeded 60%, the patient was categorized as “improved.” Conversely, if the percentage value was less than 0%, the patient situation was deemed to have “worsened”. In cases where the percentage fell between 0% and 60% and the clinical manifestations remained unchanged, the patient situation was considered “unchanged”. Paired-sample t-test was performed to examine the effectiveness of treatment on the quality of life.
Objective clinical monitoring
The clinical assessments were conducted before and four weeks after the initiation of therapy. The AChR binding antibody was tested using AChR-Ab ELISA assay described in [33] in order to monitor the antibody titer and track the progression of the disease as well as the response to therapy [34].
The RBC-AChE was measured using a colorimetric modified Ellman’s method [35]. In summary, the RBC-AChE assay was conducted by collecting 2 ml of whole venous blood samples into a tube containing 20 µl of EDTA at a concentration of 3.0 mg/ml. Then, the whole blood samples were centrifuged at a speed of 2500 rpm for 10 minutes, and the plasma plus cut-off was discarded. The red cell packet underwent a washing process involving three cycles of sodium phosphate buffer 0.1 M, pH 7.4. The buffer solution was prepared by dissolving 14.24 g (80 mM) Na2HPO4.2H2O and 2.4 g (17.63 mM) NaH2PO4 in 800 ml of deionized water. pH level was achieved by adding 0.1 N HCl or NaOH. Centrifugation was performed between each wash step, as previously outlined, until a clear and colorless supernatant was obtained.
A total of 0.8 ml of red-washed pellet was divided into two tubes, designated A and B. Tube A contained 1.2 ml of a solution consisting of 1% Triton X-100 in PBS and 0.1% NaN3, while tube B contained 1.2 ml of a solution consisting of PBS and 0.1% NaN3. The pellet was then resuspended in 1.2 ml of a solution consisting of 1% Triton X-100 in PBS and 0.1% NaN3, which was placed in another tube containing 1.2 ml of a solution consisting of 0.1% NaN3-PBS. In tube A, the red blood cells underwent lysis, resulting in the solubilization of the membrane and the subsequent release of free acetylcholinesterase (AChE). A small quantity of insoluble debris was present, which was eliminated through centrifugation in microcentrifuge tubes for a duration of 30 minutes at 10,900 rpm (12000 g). In test tube B, the suspension was utilized to measure the total hemoglobin levels using an automated hematological analyzer (Orpheé Mytic 18 – C2 Diagnostic France). The results were expressed in grams per deciliter (g/dL).
The solution containing solubilized no-ghost RBC-AChE exhibits a red coloration but does not possess a high viscosity, which renders it suitable for use in the AChE assay. A volume of 5 µl of no-ghost RBC-AChE solutions was combined with 1.98 ml of DTNB solution, which consisted of 0.5 mM 5,5’-dithiobis-2-nitrobenzoic acid in 0.1 M phosphate buffer (9.34 g/L potassium phosphate dibasic and 6.31 g/L potassium phosphate monobasic in sterile dH2O) containing 1.5 mg/ml sodium bicarbonate and adjusted to pH 7.0. This mixture was incubated for 10 minutes to deplete any free sulfhydryl groups. Following this preincubation, the AChE reaction with acetylthiocholine iodide (ACTI) was initiated by adding 20 µl of a 0.075 M ACTI substrate solution in 0.1 M phosphate buffer (16.28 g/L of potassium phosphate dibasic and 0.89 g/L potassium phosphate monobasic in sterile dH2O) pH 8.0.
The colorimetric response was assessed by monitoring the kinetic increase in absorbance (Abs) at a wavelength of 436 nm using a spectrophotometer (Clinicon 4010 – Boehringer Germany) for a duration of 4 minutes. Subsequently, the rate of change in absorbance per minute (ΔAbs/min) was computed, but only in cases where linear kinetics were observed. The activity of acetylcholinesterase (AChE) was determined using the molar absorption coefficient of 11280×mol-1×cm-1 for the dianion of 5-thio-2-nitrobenzoic acid [35]. This value was adjusted to account for the hydrolysis of the substrate in the absence of AChE (blank). The AChE activity was then quantified in units per milliliter (U/ml) of the red blood cell (RBC)-AChE solution, using the subsequent formula:
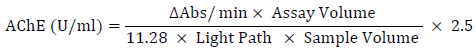
Where:
ΔAbs/min: The change in absorbance per minute
Assay Volume: Total reaction volume (ml) (2.005 ml)
11.28: absorbance coefficient of 5-thio-2-nitrobenzoic acid at 436 nm
Light Path: Length of the light path (cm) (1 cm)
Sample volume: No-ghost RBC-AChE sample volume expressed (ml) (0.005 ml)
2.5: Dilution factor of the preliminary sample
The activity value of RBC-AChE (Q) was then estimated by standardizing AChE activity against whole blood hemoglobin using the following formula:
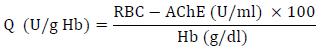
In prior studies investigating hazardous acetylcholinesterase (AChE) inhibitors, the Q value was divided into the “inhibition without symptoms” category, which exhibits values ranging from 24.5 to 31.3 U/g Hb and the “severe inhibition” category, defined by values below 24.5 U/g Hb, which aligns with the designated cutoff point [36].
Although all patients (except for one) were already taking AChE inhibitors prior to the therapy program, Q levels were assessed in the patients before and four weeks after initiating the study protocol. The objective was to determine whether the inhibition of acetylcholinesterase (AChE) reached therapeutic levels, namely within the range of 25-35 U/g Hb. Additionally, the presence of anti-AChR antibodies (AChR-Ab) was measured before treatment initiation and after six months of the treatment.
Results
Myasthenia gravis case description
Case #1, LPI (F, 43 Y, MG class 2): The commencement of symptoms occurred three years before treatment initiation and included left eyelid ptosis, impaired left ocular movement, and limb weariness. The post-diagnosis treatment involved the administration of PYR-B at a dosage of 60 mg every 3 hours, necessitating ongoing changes to maintain the patient’s ability to engage in regular daily activities. After one year, following an initial two-month period of weaning with HupA (100 mcg/bid) and PYR-B (15 mg/bid) which was subsequently reduced to 7.5 mg/bid, the treatment regimen exclusively consisted of HupA (200 mcg/od). This treatment resulted in the maintenance of muscle functions for approximately five hours, followed by a gradual decrease. In contrast, the effects of PYR-B decreased rapidly, depending on the level of physical activity undertaken. The observed favorable effect did not persist for an extended duration, prompting the need for an additional administration of HupA (100 mcg) in the evening when increased fatigue was experienced. Notably, no adverse effects of the PYR-B variety were recorded. Nevertheless, throughout the duration of the patient’s observation period, it was periodically necessary to make adjustments to the treatment regimen by increasing the dosage of PYR-B to 15 mg per day. This was deemed necessary due to the inadequacy of HupA in sustaining muscle strength.
Case #2, LPI (F, 74 Y, MG class 3): presented two years before treatment initiation with symptoms including reduced muscle strength, drooping of the eyelid (ptosis), double vision (diplopia), and speech impairments. Following diagnosis, the treatment with PYR-B at a dosage of 60 mg every four hours was initiated. Following a duration of one week, a progression of the initial symptoms, including weakness, dysarthria, aphasia, dysphagia, bowel motions, and diarrhea were noticed. Additionally, there an onset of agitation, confusion, anxiousness, irritability, bradycardia, hypotension, and bronchospasm occurred. The second symptom has been ascribed to the patient’s co-existing intrinsic asthma, whereas the clinical presentation suggests an iatrogenic cholinergic crisis that imitates the disease. This occurrence is not uncommon when administering AChEI medications, as their symptoms might be mistaken for those of MG. Over the course of the previous year, the patient adhered to the prescribed regimen of HupA at a dosage of 100 mcg/bid and PYR-B at a dosage of 7.5 mg/tid, without experiencing any adverse effects or requiring any adjustments to the doses.
Case #3, LPI (M, 39 Y, MG class 2): The patient had the onset of symptoms one year before treatment initiation, characterized by ptosis, muscular weakness affecting the eyes, diplopia, and pronounced weakness in the limbs, leading to a limited ambulation capacity of merely 50 meters. Following the diagnosis, the patient was administered PYR-B at a dosage of 30 mg every 3 hours, resulting in the ability to ambulate a distance of 100 meters. However, after a duration of 4 days, the patient had adverse effects of such severity that necessitated a substitution of PYR-B with prednisone at a daily dosage of 25 mg. Hyperglycemia manifested as a result of corticotherapy, and despite the implementation of several therapeutic interventions aimed at mitigating its effects, such as lifestyle modifications, administration of oral hypoglycemic medications, and ultimately insulin therapy, the management of blood glucose levels remained suboptimal. Given the persistent nature of ocular motility and diplopia correction, it became necessary to escalate the dosage of CS. During the subsequent phase, the patient adhered to the prescribed regimen of HupA 200 mcg/bid in combination with 5 mg/od of prednisone, experiencing only a limited number of moderate side effects, notably headaches. However, these headaches resolved after one week of initiating the medication.
Case #4, (M, 33 Y, MG class 2): The patient presented with the initial symptoms of a drooping eyelid (which occurred 8 months before treatment initiation) and subsequent limb weakness. The ocular fissure measurements indicated a left ocular fissure of 5 mm and a right ocular fissure of 2 mm. Initially, the patient was prescribed a dosage of PYR-B at 15 mg every 4 hours. However, it was necessary to adjust the dosage during the day due to the patient experiencing significant fatigue, particularly in the afternoon and evening. The tested treatment protocol was then initiated with the administration of HupA at a dosage of 100 mcg/bid, in conjunction with PYR-B at a dosage of 15 mg/od. This combination was effective in achieving satisfactory symptom control. Consequently, after a duration of 4 months on this medication, the patient transitioned to a monotherapy regimen consisting of HupA at a dosage of 100 mcg in the morning and 200 mcg in the afternoon. The experimental protocol facilitated the observation that HupA exhibited a more gradual initiation and decline in comparison to PYR-B. This characteristic enabled the patient to effectively regulate their chronopharmacokinetics [37].
Case #5, (F, 41 Y, MG class 2): The onset of generalized weakness occurred two years before treatment initiation, primarily affecting the arms. This weakness has gradually worsened, particularly in the late morning following manual office labor. However, there was a minor improvement noted during the lunch break. Engaging in household activities at exacerbated fatigue in the arms, leading to the manifestation of blurred vision while performing computer-related tasks. The initial medication suggested, PYR-B 30 mg every three hours, resulted in a notable enhancement in the symptomatic presentation. Nevertheless, during the course of the past year, a range of symptoms associated with lower urinary tract (LUT) dysfunction have manifested, initially presenting as moderate and subsequently progressing in severity until reaching a highly severe state. The patient presented with symptoms of polyuria, including nocturia and pollakiuria, accompanied by urine urgency and moderate overactive bladder incontinence (OAB). Indeed, there was a valid association between overactive bladder (OAB) and myasthenia gravis (MG). However, it is worth noting that this correlation could potentially be exacerbated by the use of pyridostigmine bromide (PYR-B). This medication has the capacity to stimulate the smooth muscle of the bladder, hence inducing detrusor overactivity. All blood and urinary parameters were within normal ranges. Given the presence of pre-existing lower urinary tract symptoms (LUTs), a physiatric examination of the pelvic floor was suggested, which facilitated the identification of pelvic floor descent. A rehabilitation program incorporating Kegel exercises was implemented, resulting in enhanced control. However, the efficacy of the program further improved when the patient transitioned to a treatment regimen involving HupA at a dosage of 200 mcg/bid and PYR-B at a dosage of 15 mg/bid. After a period of 3 months, the administration of PYR-B was discontinued, leaving only HupA as the acetylcholinesterase inhibitor (AChEI) in the protocol.
Case #6, (F, 37 Y, MG class 2): The patient experienced the onset of symptoms one year before treatment initiation. The patient presented with eyelid drooping and exhibited altered constriction-dilation cycles of the pupils when exposed to a stationary, discrete slit lamp beam. These cycles were found to be significantly prolonged at 1,022 milliseconds, compared to the normal value of 801.9 ± 8.6 milliseconds. Additionally, there was a correlation observed between the duration of myasthenia and the slowing of cycle time, as well as the presence of dysphagia and pronounced weakness after short periods of walking. The patient was administrated PYR-B at a dosage of 30 mg every 4 hours. However, since the initiation of the treatment, the patient has been experiencing frequent episodes of incoherent speech followed by the passage of feces. The consistency of the feces is typically classified as type 5 or 6 according to the Bristol stool chart, with occasional instances of a more liquid consistency type [38][39]. This symptom is associated with the impact of MG on smooth muscle derived from ectoderm, the neuromuscular autonomic junction, or both, and is not confined to the recognized abnormalities in the neuromuscular junction of striated muscle originating from mesoderm. Nevertheless, neuromuscular symptom was controlled by PYR-B, and the side effect imposed a reduction of PYR-B to 15 mg/bid inserting our protocol with HupA 100 mcg in the morning and 200 mcg in the afternoon.
Subjective monitoring
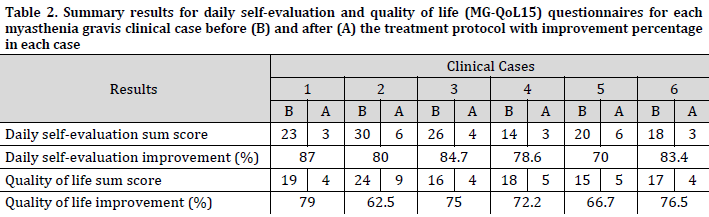
A decent decrease in the subjective symptom perception was observed with an improvement of more than 60% for all cases in both questionnaires when comparing the sum scores before and after the treatment (Table 2). The daily self-evaluation for symptoms ranged between 70 and 87% in the cases with an average of 80.6±5.5%, while the quality of life improvement results ranged between 62.5 and 79% with an average improvement of 72±5.7%. Furthermore, the paired-sample t-test showed that quality of life (QoL) improvement was significantly associated (t <0.002) with the treatment. indicating a favorable therapeutic index for the treatment program.
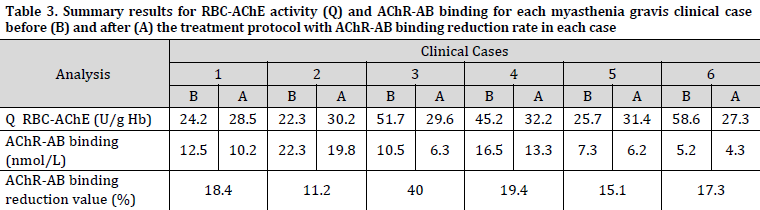
Q activity levels (RBC-AChE U/gHb) four weeks after treatment ranged between 27.3 and 32.2 with an average of 29.7±1.7 (Table 3). The serum levels of AChR-Ab exhibited a drop following therapy in all patients. Notably, Case #3, who received corticosteroid (CS) treatment, demonstrated a considerable reduction in AChR-Ab levels, which can be attributed to the immunosuppressive properties of corticotherapy. The reduction value in AChR-Ab titer ranged from 11.2 to 40 with an average of 20.2±9.2 and it exhibited a moderate positive correlation (R2=0.4) with a decrease percentage in clinical symptoms.
Discussion
Until recently, the primary objective of myasthenia gravis (MG) therapy has primarily revolved around alleviating the various symptoms associated with the condition. These symptoms encompass weakness in the extremities, difficulties in swallowing, impaired speech, drooping eyelids, and visual disturbances such as double or blurred vision. However, there has been a recent shift in focus towards not only mitigating symptom burden but also minimizing the challenges posed by the treatment itself, a matter that poses significant issues for numerous individuals afflicted with MG. If there is a possibility of decreasing death rates associated with MG through the enhancement of overall muscle strength, it is important to acknowledge that a significant proportion of MG patients still experience limitations in physical vitality and endurance, which may hinder their ability to fully engage in and get satisfaction from various aspects of life. When selecting the therapeutic regimen, it is essential to consider not only the etiopathogenesis and clinical stage of the disease but also the comorbidities reported by the patient, as well as the potential side effects associated with the prescribed medications. This comprehensive evaluation is crucial in order to develop a highly individualized treatment plan.
A decrease in the subjective symptom perception especially in the common symptoms such as weakness after short walking (Supplementary Table 1) and having trouble walking (Supplementary Table 2) was observed. This decrease resulted in an overall improvement beyond 70%, providing evidence that the implemented protocol has been effective in enhancing the patient’s performance. However, it is important to note that complete elimination of the clinical symptoms has not yet been achieved. Despite a strong correlation (t< 0.002) observed in the outcomes of the clinical/psychosocial questionnaires, the quality of life (QoL) data shows lower percentage outcomes. This discrepancy may be attributed to the individuals’ awareness of being caught in a profound emotional conflict associated with the disease. Furthermore, the lack of established therapeutic interventions for this chronic disease likely contributes to the inability to effectively manage its impact on the patients’ well-being.
In a previous study examining the pharmaceutical treatments administered to patients with MG, it was shown that PYR-B was prescribed as a standalone therapy in 53.1% of cases. Prednisolone was utilized in 21.7% of cases, while other immunomodulators were employed by 30.2% of patients. A total of 74.8% of the patients reported the use of medications for other comorbidities, while 43.4% had prescriptions that had the potential to exacerbate their symptoms. PYR-B was administered as a standalone treatment at dosages that were sufficient to ascertain the occurrence of a cholinergic crisis. However, it is noteworthy that a significant number of patients were concurrently provided a medicine that had the potential to exacerbate their condition, including some agents that might potentially precipitate a myasthenic crisis [40].
The typical recommended first oral dosage of PYR-B is 15-30 mg three to four times a day. The maximum daily dose of PYR-B should not exceed 360 mg. It is important to note that greater doses are unlikely to yield any additional therapeutic benefits and may also lead to the downregulation of the cholinergic receptor. Hence, the comparison of PYR-B underdose (myasthenic crisis) and overdose (cholinergic crisis) is marked by the manifestation of escalating muscle weakness, thereby posing challenges in their differentiation based solely on symptomatic grounds. The distinction has significant importance due to the potential adverse outcomes that may arise from escalating doses of PYR-B or similar medications within the context of cholinergic crises or a refractory condition characterized by unresponsiveness. The adverse effects associated with PYR-B primarily stem from excessive dosage and can be categorized into two main types: muscarinic and nicotinic. The symptoms exhibited by individuals in the initial cohort include nausea, vomiting, diarrhea, abdominal cramps, heightened peristalsis, augmented salivation, increased bronchial secretions, bradycardia, miosis, and diaphoresis. The predominant adverse effects associated with nicotinic exposure primarily manifest as muscular cramps, fasciculation, and weakening [41].
The compound HupA, which is a sesquiterpene alkaloid, can be obtained in a highly purified form from a plant known as Chinese moss. This compound exhibits drug-like properties and acts as a potent, selective, and well-tolerated reversible inhibitor of the enzyme acetylcholinesterase (AChE). It demonstrates a higher level of selectivity towards inhibiting AChE compared to butyrylcholinesterase (BChE), and it is even more selective towards the G4 form of AChE [42].
The current results showed that HupA can be considered an adjunctive tool rather than a standalone alternative for the management of MG. This is due to its potential to reduce the required doses of PYR-B, hence mitigating or minimizing associated adverse effects, while concurrently modifying the various forms of AChE. The administration of HupA, either alone or in conjunction with PYR-B, demonstrated a notable amelioration in muscle weakness. Furthermore, the inclusion of HupA in combination with PYR-B resulted in a prolonged duration of this beneficial effect, surpassing the effects observed when PYR-B was administered alone. It is worth noting that the occurrence of muscarinic side effects, such as sweating, nausea, headache, and blurred vision, was infrequent when HupA was added to the treatment regimen. Additionally, these side effects were generally mild in nature and of short duration. No significant changes in the biochemical-hematological analytical parameters were observed in the participating patients when administered the dosages utilized in this study, as assessed during the course of therapy.
The amelioration of symptoms observed in the current HupA incorporated protocol may also be attributed to the anti-inflammatory impact induced by the abundant production of acetylcholine (ACh) in the synaptic cleft, which serves to safeguard the acetylcholine receptor (AChR) from the inflammation caused by AChR antibodies [43]. Therefore, the inclusion of immunomodulatory therapy as part of the treatment played a substantial role in achieving this objective.
Although AChR-Ab levels notably decreased in all cases, none of the patients reached normal levels of less than 0.05 nmol/L. The modest decrease in the titer of AChR-Ab (less than 20% except for Case #3, who received CS treatment) may be attributed to the decline in immune response associated with the combined effects of ACh and ancillary immunomodulatory therapy. Since a moderate positive correlation (R2=0.4) was found between AChR-Ab reduction value and the decreased percentage in MG clinical symptoms, measurement of AChR-Ab levels can be a valuable marker for confirming the presence of the disease. However, this test should always be supported by a clinical examination.
Although RBC-AChE inhibition percentages were not calculated, since all patients (except for Case #3) were already taking AChE inhibitors before regiment initiation, it was noted that Q value for all patients was regulated within the pre-set therapeutic target (25-35 U/g Hb).
Conclusions
Due to its pharmacologic efficacy as an acetylcholinesterase inhibitor and its reasonably wide therapeutic index, HupA has been clinically utilized in patients with myasthenia gravis (MG), either as a standalone treatment or in conjunction with PYR-B and immunomodulatory medications. This therapeutic approach has demonstrated the ability to effectively alleviate clinical symptoms and enhance the overall quality of life for these patients.
It is not feasible to implement a standardized fixed-dose regimen for all patients with MG due to the variability in their treatment needs, which can change throughout the day and in response to factors such as stress or infections. Additionally, each commonly prescribed conventional acetylcholinesterase inhibitor (AChEI) has its limitations, suggesting the use of a low-dose treatment approach combined with natural inhibitors. The current study demonstrates that this therapeutic approach can effectively manage the condition without causing noticeable cholinergic imbalances.
Although an overall enhancement was observed through the currently reported therapeutic protocol, the obtained results are still far from satisfactory in controlling MG. Therefore, it is necessary to consider additional therapeutic alternatives for certain patients to further enhance the response and overall performance of MG patients. “People with myasthenia are getting better, but they’re still not well”
References
| 1 | Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8(5):475-90. DOI |
| 2 | Dani JA. Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int. Rev. Neurobiol. 2015;124:3-19. DOI |
| 3 | Zoli M, Pucci S, Vilella A, Gotti C. Neuronal and extraneuronal nicotinic acetylcholine receptors. Curr. Neuropharmacol. 2018;16(4):338-49. DOI |
| 4 | Pechlivanidou M, Ninou E, Karagiorgou K, Tsantila A, Mantegazza R, Francesca A, Furlan R, Dudeck L, Steiner J, Tzartos J, Tzartos S. Autoimmunity to Neuronal Nicotinic Acetylcholine Receptors. Pharmacol. Res. 2023:106790. DOI |
| 5 | Simon T, Kirk J, Dolezalova N, Guyot M, Panzolini C, Bondue A, Lavergne J, Hugues S, Hypolite N, Saeb-Parsy K, Perkins J. The cholinergic anti-inflammatory pathway inhibits inflammation without lymphocyte relay. Front. Neurosci. 2023;17:1125492. DOI |
| 6 | Reardon C, Duncan GS, Brüstle A, Brenner D, Tusche MW, Olofsson PS, Rosas-Ballina M, Tracey KJ, Mak TW. Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proc. Natl. Acad. Sci. 2013;110(4):1410-5. DOI |
| 7 | Lu J, Wu W. Cholinergic modulation of the immune system–A novel therapeutic target for myocardial inflammation. Int. Immunopharmacol. 2021;93:107391. DOI |
| 8 | Zivkovic AR, Paul GM, Hofer S, Schmidt K, Brenner T, Weigand MA, Decker SO. Increased Enzymatic Activity of Acetylcholinesterase Indicates the Severity of the Sterile Inflammation and Predicts Patient Outcome following Traumatic Injury. Biomolecules. 2023;13(2):267. DOI |
| 9 | de Almeida JL, Saldanha C. Nonneuronal cholinergic system in human erythrocytes: Biological role and clinical relevance. J. Membr. Biol. 2010;234:227-34. DOI |
| 10 | Carvalho FA, Almeida JP, Fernandes IO, Freitas-Santos T, Saldanha C. Non-neuronal cholinergic system and signal transduction pathways mediated by band 3 in red blood cells. Clin. Hemorheol. Microcirc. 2008;40(3):207-27. DOI |
| 11 | Silver A. The biology of cholinesterases. Frontiers of biology. North Holland Publishing Company. 1974;36:58-67. |
| 12 | Saldanha C. Human erythrocyte acetylcholinesterase in health and disease. Molecules. 2017 Sep 8;22(9):1499. DOI |
| 13 | Shao X, Yang L, Hu K, Shen R, Ye Q, Yuan X, Zhao Q, Shen J. Serum cholinesterases, a novel marker of clinical activity in inflammatory bowel disease: a retrospective case-control study. Mediators Inflamm. 2020;2020. DOI |
| 14 | Jacob S. Myasthenia gravis—A review of current therapeutic options. Eur. Neurol. Rev. 2018;13:86-92. DOI |
| 15 | Westerberg E. Environmental Factors of Importance in Myasthenia Gravis: Emphasis on Physical Activity. Doctoral dissertation, Acta Universitatis Upsaliensis. 2018. |
| 16 | Farmakidis C, Pasnoor M, Dimachkie MM, Barohn RJ. Treatment of myasthenia gravis. Neurol. Clin. 2018;36(2):311-37. DOI |
| 17 | Farrugia ME, Goodfellow JA. A practical approach to managing patients with Myasthenia Gravis—Opinions and a review of the literature. Front. Neurol. 2020;11:604. DOI |
| 18 | Friedli MJ, Inestrosa NC. Huperzine A and its neuroprotective molecular signaling in Alzheimer’s disease. Molecules. 2021;26(21):6531. DOI |
| 19 | Feaster SR, Gordon RK, Doctor BP, inventors; US Department of Army, assignee. Assay for detecting, measuring and monitoring the activities and concentrations of proteins and methods of use thereof. United States patent US 6,746,850. 2004. |
| 20 | Petrov KA, Kharlamova AD, Lenina OA, Nurtdinov AR, Sitdykova ME, Ilyin VI, Zueva IV, Nikolsky EE. Specific inhibition of acetylcholinesterase as an approach to decrease muscarinic side effects during myasthenia gravis treatment. Sci. Rep. 2018;8(1):304. DOI |
| 21 | Yu CM, Tang XC, Liu JS, Han YY. Huperzines and analogs. United States Patent US 5,177,082. 1993. |
| 22 | Erdogan Orhan I, Orhan G, Gurkas E. An overview on natural cholinesterase inhibitors-a multi-targeted drug class-and their mass production. Mini-Rev. Med. Chem. 2011;11(10):836-42. DOI |
| 23 | Wang R, Yan H, TANG XC. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol. Sin. 2006;27(1):1-26. DOI |
| 24 | Jaretzki A, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, Sanders DB. Myasthenia gravis: recommendations for clinical research standards1. Ann. Thorac. Surg. 2000;70(1):327-34. DOI |
| 25 | Park Y, Lee A, Shim SC, Lee JH, Choe JY, Ahn H, Choi CB, Sung YK, Bae SC. Effect of n-3 polyunsaturated fatty acid supplementation in patients with rheumatoid arthritis: a 16-week randomized, double-blind, placebo-controlled, parallel-design multicenter study in Korea. J. Nutr. Biochem. 2013;24(7):1367-72. DOI |
| 26 | Cadegiani FA. Remission of severe myasthenia gravis after massive-dose vitamin D treatment. The American journal of case reports. 2016;17:51-4 DOI |
| 27 | Wang S, Li H, Zhang M, Yue LT, Wang CC, Zhang P, Liu Y, Duan RS. Curcumin ameliorates experimental autoimmune myasthenia gravis by diverse immune cells. Neurosci. Lett. 2016;626:25-34. DOI |
| 28 | Kleemann R, Verschuren L, Morrison M, Zadelaar S, van Erk MJ, Wielinga PY, Kooistra T. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011;218(1):44-52. DOI |
| 29 | Thomas P, Evans C. An identity crisis? Aspects of patient misidentification. J. Patient Saf. Risk Manag. 2004;10(1):18-22. DOI |
| 30 | Burns TM, Conaway MR, Cutter GR, Sanders DB, Muscle Study Group. Less is more, or almost as much: a 15‐item quality‐of‐life instrument for myasthenia gravis. Muscle Nerve. 2008;38(2):957-63. DOI |
| 31 | Burns TM, Cutter G, Nagane Y, Murai H, Masuda M, Farrugia ME, Carmichael C, Birnbaum S, Hogrel JY, Nafissi S, Fatehi F, Ou C, Liu W, Wolfe G, Silvestri NJ, Gable KL, Massey J, Sadjadi R, Hobson-Webb L, Utsugisawa K, Gwathmey KG, Barnett C, Joshi A, Jones S, Bril V, Guptill JT, Sanders DB, Juel VC, Conaway M. International clinimetric evaluation of the MG‐QOL15, resulting in slight revision and subsequent validation of the MG‐QOL15r. Muscle Nerve. 2016;54(6):1015-22. DOI |
| 32 | Dewilde S, Philips G, Paci S, Beauchamp J, Chiroli S, Quinn C, Day L, Larkin M, Palace J, Berrih-Aknin S, Claeys KG, Muppidi S, Mantegazza R, Saccà F, Meisel A, Bassez G, Murai H, Janssen MF. Patient-reported burden of myasthenia gravis: baseline results of the international prospective, observational, longitudinal real-world digital study MyRealWorld-MG. BMJ open. 2023;13(1):e066445. DOI |
| 33 | Hewer R, Matthews I, Chen S, McGrath V, Evans M, Roberts E, Nute S, Sanders J, Furmaniak J, Smith BR. A sensitive non-isotopic assay for acetylcholine receptor autoantibodies. Clin. Chim. Acta. 2006;364(1-2):159-66. DOI |
| 34 | Lazaridis K, Tzartos SJ. Autoantibody specificities in myasthenia gravis; implications for improved diagnostics and therapeutics. Front. Immunol. 2020;11:212. DOI |
| 35 | Eyer P, Worek F, Kiderlen D, Sinko G, Stuglin A, Simeon-Rudolf V, Reiner E. Molar absorption coefficients for the reduced Ellman reagent: reassessment. Anal. Biochem. 2003;312(2):224-7. DOI |
| 36 | Mamadou A, Doumma A, Mazih A, Coulibaly BM. Exposition aux organophosphorés en milieu rural nigérien: étude de l’activité enzymatique érythrocytaire des cholinestérases comme indicateur biologique. VertigO. 2008;8(3). DOI |
| 37 | Bicker J, Alves G, Falcão A, Fortuna A. Timing in drug absorption and disposition: The past, present, and future of chronopharmacokinetics. Br. J. Pharmacol. 2020;177(10):2215-39. DOI |
| 38 | Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997;32(9):920-4. DOI |
| 39 | Bacci ED, Coyne KS, Poon JL, Harris L, Boscoe AN. Understanding side effects of therapy for myasthenia gravis and their impact on daily life. BMC Neurol. 2019;19:1-3. DOI |
| 40 | Machado‐Alba JE, Calvo‐Torres LF, Gaviria‐Mendoza A, Augusto MejíA‐Vélez C. Prescription profile of pyridostigmine use in a population of patients with myasthenia gravis. Muscle Nerve. 2017;56(6):1041-6. DOI |
| 41 | Harjana LT, Hardiono H. Myasthenia Crisis Vs Cholinergic Crisis: Challenges in Crisis Management Without Plasmapheresis or Intravenous Immunoglobulin (IVIG). Indones. J. Anesth. Reanimat. 2020;2(2):53. DOI |
| 42 | Zhao Q, Tang XC. Effects of huperzine A on acetylcholinesterase isoforms in vitro: comparison with tacrine, donepezil, rivastigmine and physostigmine. Eur. J. Pharmacol. 2002;455(2-3):101-7. DOI |
| 43 | Wang J, Chen F, Zheng P, Deng W, Yuan J, Peng B, Wang R, Liu W, Zhao H, Wang Y, Wu G. Huperzine A ameliorates experimental autoimmune encephalomyelitis via the suppression of T cell-mediated neuronal inflammation in mice. Exp. Neurol. 2012;236(1):79-87. DOI |
Cite this article:
Zagami, F., Alabboud, M. Myasthenia gravis symptom response to huperzine A, pyridostigmine bromide, and an immunomodulatory incorporated regimen: A multi-case study. DYSONA – Life Science, 2023;(4)2:36-49. doi: 10.30493/dls.2023.789777
