Abdelhak Rhouma 1*; Lobna Hajji-Hedfi 1; Khaled Atallaoui 2
1, Regional Centre of Agricultural Research of Sidi Bouzid, CRRA, Gafsa Road Km 6, B.P. 357, 9100, Sidi Bouzid, Tunisia
2, Department of Natural and Life Sciences, University of Djelfa, Algeria
E-mail:
abdelhak.rhouma@gmail.com
Received: 23/02/2024
Acceptance: 22/03/2024
Available Online: 23/03/2024
Published: 01/04/2024

Manuscript link
http://dx.doi.org/10.30493/DLS.2024.445326
Abstract
After almost two centuries since the infamous Irish Potato Famine, potato late blight, caused by the oomycete Phytophthora infestans, continues to be the most economically destructive potato disease globally, resulting in annual losses surpassing 10 billion USD. Considerable advancements have been made over several decades in comprehending the molecular mechanisms behind the pathogenicity of P. infestans and devising efficient ways for its management. However, in order to guarantee food security for an expanding global population in the face of a changing environment, it is imperative to make more progress in developing effective, sustainable, and economically flexible strategies to control potato late blight. This work delves into the complex nature of P. infestans, reviews the effectiveness of current integrated pest management strategies, and analyzes the potential of innovative sustainable ways for controlling the disease.
Keywords: Phytophthora infestans, Potato late blight, Management practices, Sustainability
Introduction
Potato late blight, caused by the oomycete pathogen Phytophthora infestans, inflicts devastating losses to potato globally, with its greatest significant outbreak in history leading to the Irish Potato Famine of the 1840s [1]. Having originated in Central Mexico or South America, it has subsequently expanded its presence to major potato-producing countries such as the US, Canada, China, and India [2].
Currently, potato late blight continues to be the most serious biological limitation to potato production worldwide, presenting a significant danger to food security, particularly in places that heavily depend on potatoes as a primary source of food [3]. According to estimates, the yearly global losses in potato crop and the expenditures associated with managing potato late blight are estimated to be between 3 and 10 billion USD [4]. In economically constrained underdeveloped nations, the inability to afford adequate chemical control measures can lead to significant output losses of over 60% due to potato late blight [5]. Insufficient disease control in these regions leads to much lower potato yields per area unit compared to developed countries [6]. In contrast, the use of insecticides to suppress potato late blight in affluent nations might result in a loss of 10-25% of the market value of the potato harvest [7]. In certain areas, it is necessary to use pesticides up to eight times during the potato growing season in order to achieve sufficient control. This leads to considerable economic and environmental challenges associated with potato production [8].
The early symptoms of potato late blight are dark grey to brown lesions on the leaves, which appear water-soaked. These lesions are sometimes surrounded by white, mold-like growth [9]. Under optimal circumstances, the infection swiftly spreads within and among plants [3]. The pathogen’s elongated growth, along with the spread of infectious asexual sporangia through the air and water drops can result in the rapid death of infected plants within a few days [10]. Tubers are susceptible to damage either through the spread of pathogens across the entire system or from the washed off sporangia harboring conidia. Infection commonly initiates at vulnerable areas such as lenticels or wounds [11]. The pathogen’s endogenous proliferation within the tuber induces discoloration, frequently exacerbated by secondary infections from soft rot bacteria, resulting in the tuber being putrid and unsuitable for human or animal use [12][13].
Significant progress has been made in the management of potato late blight in recent decades [5]. Growers and researchers have effectively utilized local epidemic forecasting systems and focused chemical control methods to optimize field management [13]. In addition, extensive molecular and genomic studies have shown the complexities of the relationship between P. infestans and its host. This has brought new possibilities for the development of advanced, eco-friendly methods of controlling the disease [14].
Integrated pest management (IPM) is a promising method that aims to achieve a balance between immediate effectiveness and long-term environmental and ecological costs [5][15]. This sustainable solution differs from standard methods that prioritize blanket crop protection [4]. Effective IPM requires a thorough comprehension of pest life cycles and their interactions with hosts. This knowledge allows for precise interventions, such as the use of pesticides and adjustments in agricultural practices, at critical stages [16]. By taking these educated activities, one can assure sufficient protection with minimal interruption, hence reducing overall management expenses while preserving yield and quality [17][18].
This review examines the current understanding of the molecular pathology of P. infestans, investigates the current strategy for managing potato late blight by using effective fungicides and forecasting systems, and discusses the potential of enhancing crop resistance through cultural practices, intercropping, resistant varieties, and biological control.
Etiology of Phytophthora spp.
Anton de Bary was the first to differentiate Phytophthora from its previous classifications (as Botrytis infestans and Peronospora infestans), by examining the distinct morphology of its conidiophores, which are structures responsible for spore production [19][20]. Phytophthora may be recognized by its hyphae, which lack septa and have constrictions at the branches. It also produces ovoid sporangia on branching sporangiophores and has biflagellate zoospores. Furthermore, the presence of oogonia containing just one oospore and antheridia that are amphigynous or paragynous are distinctive features [1][20][21]. These soil-borne pathogens infect both herbaceous and woody dicots, causing root, stem, leaf, and fruit rots [1]. They spread to both cultivated and wild plants worldwide, resulting in agricultural losses and disturbances to ecosystems. Currently, there are newly discovered isolates that need to be confirmed for their ability to cause disease. However, the recent findings of P. gemini, P. inundata, and an unknown species infecting seagrass are causing worries for coastal ecosystems [22].
In the past, Phytophthora taxonomy was mostly based on morphological traits such as the shape of sporangia, the production of oospores, and the size of antheridia and oogonia using Waterhouse’s six morphological groupings [14]. Nevertheless, due to the progress made in molecular techniques, DNA sequence analysis is now more commonly used for identifying species and conducting phylogenetic research. This method has allowed for the discovery of intricate relationships within the genus and has emphasized the importance of ongoing taxonomic improvement [1][22].
Hyphal swellings, which lack partitions, can appear as individual or grouped formations, either at the sites where branches meet (terminal), inside hyphae (intercalary), or forming complex networks. Their forms range from round to oval or irregular, exhibiting significant variation in size. Although they can be observed on agar plates, their growth is enhanced in a water culture. These swellings bear a resemblance to chlamydospores, but they possess specific properties that enable distinction. Chlamydospores have a second layer of protective covering, clear boundaries between sections, and frequently show rearrangement of their internal cytoplasm. Their hue varies from transparent to yellowish or brownish, and their walls, typically measuring 1-2 μm in thickness, can be either thin or thick. Chlamydospores can be found either at the end or in the middle of the hyphae [1][22][23].
Three types of sporangia can be observed in Phytophthora depending on the shape of their apex: (1) prominently papillate with a distinct hemispherical thickening that is more than 3.5 μm, (2) somewhat papillate (semi-papillate) with a shallow thickening that is less than 3.5 μm, and (3) non-papillate with no thickening at the apex. The papillate and semi-papillate variants may possess a basal plug, but non-papillate forms may exhibit temporary “semi-papillation” prior to the release of zoospores or exposure to air [1][22][23]. Papillate and semi-papillate sporangia can form on agar or host tissues, either with or without pedicels of different lengths in water. The narrow exit holes, which are less than 7 μm in size, are generated after the dissolution of apical thickening. On the other hand, non-papillate sporangia are only found in water, lack pedicels, and have wide exit pores that are larger than 10 μm. In addition, their base remains open, allowing for a distinctive process called “internal proliferation” in which a new sporangium develops within or spreads beyond the empty one through the sporangiophore. This process can be repeated multiple times [1][22][23].
The sporangium contains zoospores that have two flagella and are discharged through a temporary vesicle. Papillate and semi-papillate sporangia accomplish this by dissolving the thickening at the tip, while non-papillate ones expand their apex. The ovoid zoospores, measuring 10-17×7-12 μm, utilize two flagella for propulsion: a shorter flagellum at the front for movement and a longer flagellum in the back that functions as a rudder. They display a helical swimming pattern as they rotate in a clockwise direction around their axis [1][22][23].
Zoospores retain their ability to move for a maximum of 24 hours in water before transforming into spherical structures (with a diameter of 7-14 μm) surrounded by a cell wall, while shedding their flagella. The encysted zoospores have the ability to germinate in multiple ways, with or without a germ sporangium, through the emergence of another zoospore, or straight from the sporangium by one or more germ tubes near the apex or sporangiophore [1][22][23].
Due to progress in molecular biology and automation, taxonomic notions have undergone changes utilizing both morphological and DNA fingerprints to identify the specimens [24]. Websites such as PhytophthoraDB and Phytophthora-ID.org were created as a result of endeavors focused only on the development of molecular-based identification methods. In addition, taxonomy classifications transitioned from Waterhouse’s artificial morphological categories to clades that accurately represent natural relationships using DNA data. Researchers identified eight clades [25][26], which were then expanded to ten [11].
Prior to the 1980s, the classification of Phytophthora and other oomycetes within the fungal kingdom was mistakenly based only on morphology [20]. In the 19th century, there were first attempts to classify these species with real fungus, based on surface-level similarities such as mycelia and spores. Despite early hints of differences (e.g., algal similarities) and the introduction of the term “oomycete” in 1880, the perceived phylogenetic proximity to fungi persisted [27]. Ultimately, differences in metabolic pathways [1] and distinct cell wall composition [20] led to the establishment of a distinct kingdom, Chromista, which includes oomycetes and other creatures resembling fungi [19]. Advancements in molecular phylogenetics revealed an even wider gap between fungi and oomycetes than previously thought, questioning even Chromista’s suitability, as it excluded closely related colourless oomycetes and protists [28]. The term “stramenopiles” was suggested [29] and has been widely accepted, but there is still ongoing debate on whether it should replace or be used in addition to the “Chromista” [19]. Another innovative solution was proposed categorizing the Eukaryota domain into supergroups and creating the SAR supergroup, which consists of Stramenopiles, Alveolata, and Rhizaria [14]. This classification system not only categorizes various eukaryotes, but also elucidates the separate evolutionary trajectories of oomycetes and fungi, emphasizing their relationship with algae and distinguishing them from animals (Metazoa) as well as fungi [30].
Populations of Phytophthora infestans in the world
The late blight, which originated in Central and South America, entered the United States in 1843 and caused significant damage as it spread over the East Coast. The potato blight spread relentlessly across the Atlantic, reaching Belgium in 1845 and causing widespread destruction of potato fields in France, Switzerland, Great Britain, Ireland, and Scotland. Subsequently, late blight has emerged as a recurring menace in several regions of Europe, intermittently causing substantial harm to potato cultivation. This historical narrative highlights the enduring difficulties that this disease presents to worldwide potato cultivation, requiring continuous study and management endeavors to alleviate its consequences [14][19][27][31-33].
The A2 mating type of Phytophthora infestans, which was first discovered in Mexico, began spreading globally in 1981 when it was detected in Switzerland. This spread was presumably helped by the importation of potatoes from Mexico in 1977 [30]. Furthermore, the disease introduction to England was linked to the importation of potatoes from Egypt [20]. This highly invasive disease has spread continuously across continents, making subsequent appearances in Asia [28], Africa [34], Europe [35], and North and South America [36] (Table 1).
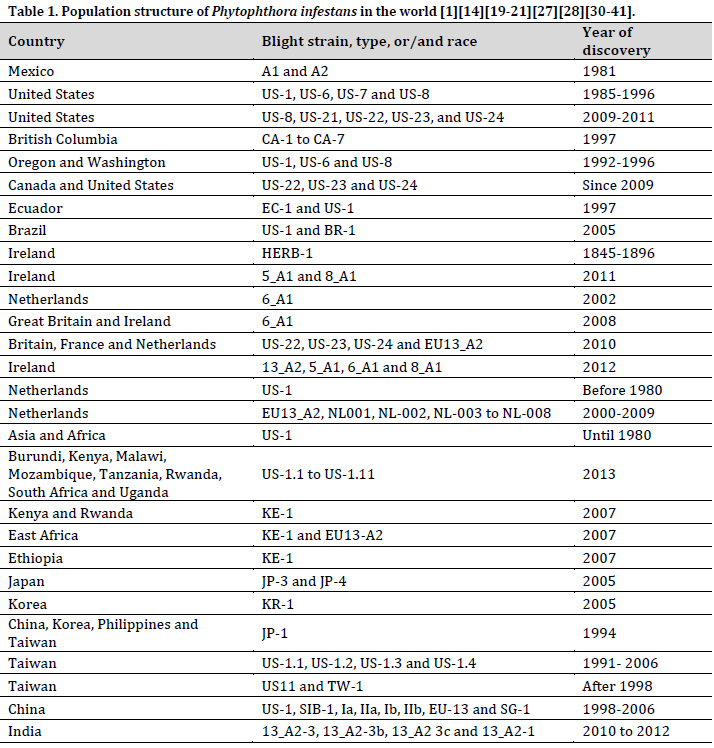
Genetic study verifies that the worldwide spread of P. infestans A2 isolates during the 1980s is not due to their natural presence or local alterations, but rather indicates a broad migration. This underscores the alarming ease with which this pathogen can cross geographical borders, underlining the necessity for strong international biosecurity measures and coordinated management methods to reduce its worldwide impact on potato production [37].
The identification of both A1 and A2 mating types of P. infestans in different places around the globe has caused concern regarding the possibility of sexual reproduction and the development of isolates with new genetic traits [38]. This issue arose when reports of oospore formation and the dormant structures resulting from sexual reproduction were observed in Europe [39] and North America [38]. Although oospore development was reported in controlled surroundings in Japan under artificial conditions, the creation of progeny was not detected [21]. These findings emphasize the possibility of sexual reproduction occurring in natural environments, emphasizing the need for additional research on how often it occurs, the environmental factors that trigger it, and its impact on virulence evolution.
Recent surveys reveal alarming patterns of swift population migrations in P. infestans across different locations. Between 2009 and 2011, a variety of mating types, including US-8, US-21, US-22, US-23, and US-24, were found in the eastern and midwestern regions of the United States [32]. In 2011, Ireland experienced a significant decrease in the prevailing 13_A2 genotype, which was replaced by the 5_A1 and 8_A1 genotypes [31]. Meanwhile, there was a growing occurrence of 6_A1 in Great Britain [31].
During the period from 1998 to 2006, China exhibited a unique situation where native genotypes coexisted with the US-1 (a globally widespread A1 strain) and SIB-1 (also known as JP-2, a pan-Eurasian A1 strain) [40]. Taiwan has also identified the presence of US-11 (US A1 genotype) in potato crops [14]. The prevalence of SIB-1 in far eastern Russia and the possibility of trans-border migration have also caused growing worries [40][41].
These findings collectively reveal the remarkable dynamism of P. infestans populations, driven by both local mutations and international migration. This underscores the challenges posed by this rapidly evolving pathogen and necessitates global collaboration in monitoring, research, and management strategies. Understanding the factors driving these population shifts, including mutation rates, environmental influences, and migration pathways, is crucial for developing effective and adaptive disease control measures to protect potato crops worldwide.
Host range
Phytophthora infestans is widely known for its devastating effects on potato and tomato crops, resulting in substantial economic losses and shortages of food. In addition to potatoes and tomatoes, P. infestans has the ability to infect other plants belonging to the Solanaceae family. This includes bell peppers (Capsicum annuum), eggplants (Solanum melongena), and even some ornamental plants like certain petunia varieties (Petunia spp.), which can also be infected [3][42].
The pathogen is thought to have originated in the Andes of South America, where it underwent co-evolution with wild Solanum species. These untamed counterparts can act as reservoirs for the disease, consequently contributing to its persistence and dissemination. The capacity of P. infestans to invade various Solanaceous plants is a crucial determinant in its ongoing predominance, as differences in the pathogen population may impact its ability to infect diverse host species and overcome host resistance [2][3].
Symptomatology
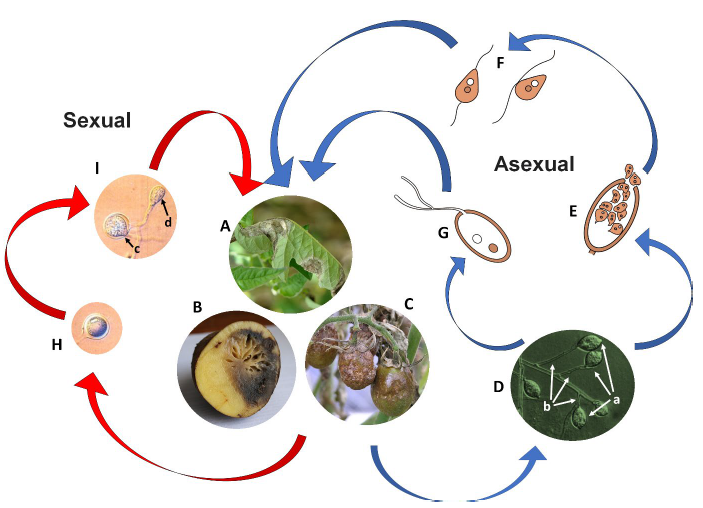
Late blight manifests through distinct visual symptoms on leaves and stems [43]. Initial signs include small, blackish/brown lesions that appear water-soaked or have chlorotic borders. These rapidly expand, engulfing the entire leaf and turning it necrotic [44]. Under humid conditions, P. infestans produces characteristic white sporulation visible at the margins of lesions on the undersides of leaves (Fig. 1 A) [3]. These structures, sporangia and sporangiophores, allow the pathogen to propagate rapidly and spread the disease [45].
Potato tubers face additional risk of late blight infection beyond foliage damage [32]. Sporangia can be washed from infected leaves and enter the soil, reaching and infecting tubers through natural openings like cracks or lenticels [46]. Early signs of tuber infection include discoloration of the affected tissues, turning them copper brown, reddish, or purplish [47]. In storage or on discarded piles, infected tubers can develop sporulation on their surface, further perpetuating the disease cycle [33]. These infections often attract and facilitate the colonization of soft rot bacteria, which rapidly decompose healthy potato tissue neighboring the initial infection site (Fig. 1 B) [48]. This decomposition results in a smelly, rotten mass that needs immediate disposal to prevent further spread [5].
Economic importance
The economic impact of late blight of potato is substantial, placing considerable financial strain on farmers and affecting potato production worldwide [12]. The disease causes significant damage to crops, resulting in decreased yields and ultimately leading to financial losses for farmers [6]. The expenses related to disease management, such as fungicides and labor, also add to the financial strain [49]. Furthermore, the presence of late blight can have negative impacts on international trade, as countries may impose restrictions to prevent the spread of the disease [4]. The storage and processing industries also face challenges that impact the availability and quality of potatoes for consumers [50]. In addition to impacting individual farms, the economic consequences of late blight also raise concerns about food security, leading to investments in research and development for resistant varieties [18]. To tackle the economic impact, it is crucial to develop a holistic strategy that encompasses efficient disease management, extensive research, and strong international cooperation. This approach will not only protect the livelihoods of farmers but also ensure global food security [2].
One of the most well-known instances of late blight in history led to the devastating Irish Potato Famine (1845-1852). The disease had a devastating impact on the Irish population, causing widespread starvation and disease. The crisis was exacerbated by British policies, resulting in approximately one million deaths and another million people forced to emigrate [49][33]. The Famine serves as a poignant reminder of the critical nature of food security and the intricate relationship between agriculture, disease, and social factors [12].
Pathogen biology
First identified as Botrytis infestans by M.J. Berkeley in the 1840s, the late blight pathogen received its current name, Phytophthora infestans, from Anton de Bary in 1876. The name itself serves as a grim reminder of its destructive nature, with “Phytophthora” meaning “plant destroyer” [52]. Contrary to traditional classification, P. infestans belongs to the oomycetes, a group more closely related to brown algae than true fungi [53]. This divergence arises from their distinct cell wall composition and genetic makeup [11]. Notably, oomycetes possess diploid nuclei (as opposed to the haploid state in most fungi) and exhibit a coenocytic mycelium lacking the extensive cellular compartments found in true fungi [14].
The key to unlocking P. infestans‘ life cycle lies in its diploid nature. This unique characteristic differentes it from fungal counterparts [54]. Belonging to the Peronosporaceae family within the Stramenopila kingdom, oomycetes diverged from fungi despite sharing certain biological, ecological, and epidemiological features with fungal plant pathogens [32]. This deeper understanding of P. infestans‘ taxonomic position and life cycle unveils crucial insights into its behavior and potential vulnerabilities, aiding in the development of more targeted and effective disease management strategies [55].
Asexual reproduction
Phytophthora infestans possesses a unique reproductive strategy involving sporangia and sporangiophores. These “sac-like” structures grow continuously (indeterminate) on stalk-like branches, promoting efficient air dispersal of the sporangia (Fig. 1 D) [1]. Interestingly, this adaptation sets P. infestans apart from most species within its genus, showcasing its specialization for airborne spread [52]. While sporangia can reach neighboring fields, their survival is limited by factors like desiccation and solar radiation, restricting long-distance travel [54]. This characteristic emphasizes the communal nature of late blight, highlighting the importance of coordinated disease management across agricultural landscapes to prevent spread [17].
Environmental conditions determine further spore development. In cool, wet conditions, zoospores emerge from the sporangia within approximately two hours (Fig. 1 E), propelled by their distinctive two flagella (biflagellate) (Fig. 1 F) [46]. These motile cells, with one tinsel flagellum for steering and one whiplash flagellum for propulsion, swim towards the host plant surface. Following encystment, they initiate infection, completing the disease cycle [48]. In warmer conditions, however, sporangia can bypass the zoospore stage, germinating directly as single spores (Fig. 1 G) [5]. This adaptability underlines the versatility of P. infestans‘ reproductive strategy, contributing to its efficiency as a plant pathogen [56].
Sexual reproduction
Under specific conditions, P. infestans can undergo sexual reproduction when both mating types, A1 and A2, encounter each other [57]. This process starts with meiosis within the gametangia, specialized structures housing gametes. Subsequently, a nucleus from the male antheridium migrates and fuses with a nucleus in the female oogonium (karyogamy). This fusion results in the formation of a thick-walled, diploid oospore capable of long-term dormancy (Fig. 1 H) [51]. The initial discovery of sexual reproduction in P. infestans occurred in Mexico, where both mating types were identified [52][58].
Disease cycle
When sexual reproduction is not involved, P. infestans primarily survives between potato cropping seasons through mycelium persistence within infected tubers or tomato fruit [3]. This underlines the critical importance of proper harvest practices to prevent leftover infected material from serving as inoculum sources [12]. Left-behind tubers, especially those discarded at field edges, can become breeding grounds for sporangia production on either the tubers themselves or newly emerging volunteer sprouts in spring (Fig. 1 B) [3]. Subsequently, these sporangia are carried by air currents, with the ability to reach and infect disease-free potato leaves [4].
Seed potatoes also pose a significant risk if contaminated. Stem lesions arising from infection can be fatal, particularly when freshly cut surfaces on seed tubers are exposed to airborne spores within storage facilities [49]. Planting infected seed potatoes can establish localized outbreaks within fields [2]. Furthermore, movement of infected tuber tissues facilitates pathogen spread, and asexual reproduction of clonal lineages further amplifies the existing inoculum population, contributing to disease severity and perpetuation through successive seasons [50].
A key factor dictating P. infestans‘ reproductive strategy is temperature. In cool, wet environments, sporangia undergo indirect germination, releasing motile zoospores armed with two flagella for efficient swimming towards host plants (Fig. 1 E and F) [3]. These zoospores encyst on the plant surface and directly penetrate, initiating infection [4]. Elevated temperatures influence P. infestans towards a streamlined infection strategy [3]. Spores (sporangia) forego the motile zoospore stage and germinate directly, forming a germ tube that invades host tissue (Fig. 1 G) [50]. Regardless of the germination method, new sporangia emerge on specialized stalk-like structures called sporangiophores within a few days after successful infection (Fig. 1 D) [25]. These deciduous sporangia readily detach, carried by wind or water to colonize new areas on the same plant or infect neighboring individuals [46]. Interestingly, P. infestans also possesses the ability to form thick-walled, dormant oospores when both mating types (A1 and A2) encounter each other [12]. These oospores can persist in soil or plant debris for extended periods, potentially contributing to disease outbreaks in subsequent seasons (Fig. 1 H) [28]. These oospores typically germinate by forming a single sporangium, perpetuating the disease cycle once favorable conditions return (Fig. 1 I) [51].
Epidemiology
Phytophthora infestans flourishes in particular climatic conditions, where humidity and temperature play crucial roles in its growth [1]. Sporulation occurs when the relative humidity falls below 90% [59]. The sporangia emerge from both infected stems and the undersides of leaves, prepared to restart the infection [12]. The method of sporangia germination is determined by temperature. Optimal temperatures, ranging from 21-26°C (70-79°F), promote immediate germination via a germ tube, completely skipping the mobile zoospore stage. Nevertheless, when temperatures drop below 18°C (65°F), the sporangia are stimulated to discharge 6-8 zoospores individually. The mobility of these cells is contingent upon the presence of water, emphasizing the reliance of late blight dissemination on moist conditions [3][55]. The ideal temperature range for sporulation is 18-22°C (64-72°F), although activity can still occur within the range of 3-26°C (37-79°F). Comprehending these environmental stimuli is essential for forecasting and controlling late blight epidemics, as well as safeguarding valuable potato crops [3][55].
Every mobile zoospore of P. infestans has the ability to start a new infection cycle [53]. This phenomenon elucidates the increased severity of diseases that is observed under settings characterized by low temperatures and high levels of precipitation [60]. Optimal conditions for late blight outbreaks occur when there is a pattern of cool nights and warm days, combined with extended periods of rain or fog [32]. In such circumstances, entire potato fields can be devastated in just a span of two weeks [46].
In addition to infecting plants, the threat also extends to storage facilities. Unregulated conditions with excessive moisture can induce the formation of spores on infected tubers [22]. Condensation subsequently forms water droplets on the surface of the tubers, creating an ideal environment for the pathogen to generate sporangia [2]. As a result, nearby tubers become contaminated, causing a chain reaction that might result in the entire storage pile being affected by soft rot bacteria [18].
Sustainable late blight management approaches
Effectively tackling late blight demands a multifaceted approach known as integrated disease management (IDM). This strategy prioritizes three key pillars: cultural practices, resistant cultivars, and strategic chemical and biological controls. By strategically combining these elements, IDM provides a comprehensive and sustainable approach to managing late blight, safeguarding potato crops, and contributing to food security. Each component plays a vital role in reducing disease pressure, minimizing yield losses, and ensuring agricultural ecosystems’ long-term health and resilience [5].
Agricultural practices
Cultural practices constitute the first line of defense against late blight, aiming to reduce the pathogen population and hinder its survival, reproduction, dispersal, and penetration of potato plants. These proactive measures play a critical role in minimizing disease outbreaks and crop losses [4]. This approach combines various practices to suppress P. infestans inoculum and limit disease establishment [4][5][46]. Planting certified disease-free seed tubers minimize pathogen introduction [5]. Removing infected plants and tubers reduces inoculum sources [46]. Proper harvesting and storing under controlled temperature/humidity limit pathogen viability [4]. Optimized irrigation practices, such as drip irrigation, avoid conditions favoring infection [5]. Maintaining good soil coverage protects tubers from soil-borne inoculum [4]. Balanced plant nutrition can enhance disease resistance [46]. Weed control ensures fungicide coverage and prevents a microclimate conducive to late blight [5]. Row orientation parallel to prevailing winds promotes foliage drying and reduces infection risk [4]. Regular inspection of stored potatoes allows for early detection and removal of infected tubers, preventing further spread [46]. By implementing these cultural and post-cultural practices holistically, farmers can significantly weaken the late blight pathogen and create a less hospitable environment for disease development. This proactive approach contributes to sustainable potato production by minimizing reliance on chemical controls and promoting overall crop health [61].
Intercropping
An interesting approach was used in Ethiopia’s central highlands, as intercropping potatoes with garlic at 3:1 ratio (75% garlic and 25% potato) showed lower P. infestans development and higher potato yield [62]. This raises the question of whether specific intercropping practices in such high-altitude settings might offer disease suppression benefits beyond the general advantages often touted for intercropping. One potential explanation lies in the physical presence of the non-host crop acting as a “bio-barrier” This barrier could physically interfere with wind and rain dispersal, potentially entrapping and reducing the available inoculum of the pathogen before it reaches and infects potato plants [43]. This phenomenon might also stem from the “dilution effect” where a higher proportion of non-host plants (garlic) reduces the available inoculum of the pathogen [49]. This, in turn, limits the formation of localized outbreaks (focal epidemics) and ultimately restricts the overall disease spread [53].
Host resistance
Host resistance holds a significant position in integrated late blight management due to its multifaceted benefits [63][64]. For farmers, resistant cultivars translate to long-term economic advantages by reducing reliance on fungicides and associated costs [58]. Additionally, this approach helps minimize shifts in the population structure of P. infestans, thereby curbing the emergence of fungicide resistance [18]. Among various control strategies, utilizing resistant varieties remains one of the most effective and environmentally sustainable methods [65]. Recognizing this importance, breeding programs for late blight resistance commenced in the 19th century and continue, albeit at a slower pace [17]. Biotechnology also offers opportunities for enhancing resistance, although genetically modified plants are incompatible with organic production systems [58]. Notably, polygenic resistance, which leverages multiple genes for disease tolerance, such as R3a, RGA2, RGA3, R1B-16, Rpi-blb2, Rpi and Rpi-vnt1 [64], demonstrates significant potential in reducing fungicide dependence [33]. Cultivars harboring polygenic resistance exhibit remarkably lower areas under disease progress curve values compared to susceptible ones, directly translating to reduced disease severity [47][64]. Continued efforts in breeding and potentially responsible application of biotechnology can further unlock the power of host resistance for sustainable and resilient potato production [63]. While both resistant potato varieties and improved cultural practices play crucial roles in mitigating late blight, their optimal application requires a nuanced understanding [17]. However, it is important to acknowledge that no variety possesses absolute immunity [59]. New pathogen strains can emerge, and even resistant cultivars may exhibit varying degrees of susceptibility under favorable disease conditions [6]. Therefore, relying solely on resistant varieties without incorporating complementary measures is not advisable [50]. Therefore, implementing effective cultural practices, such as optimized irrigation, crop rotation, and removal of infected plant debris, further disrupts the pathogen’s lifecycle and minimizes inoculum pressure, creating a less hospitable environment for disease development [45][60].
Chemical control
Strategic fungicide use can play a valuable role in protecting even resistant varieties, particularly under high disease pressure or when dealing with newly emerged pathogen strains [5]. However, the goal should be to utilize fungicides judiciously, informed by accurate disease forecasting and local regulations, to minimize environmental impact and prevent the development of fungicide resistance in the pathogen population [9]. By strategically combining resistant varieties with optimized cultural practices and targeted fungicide applications, farmers can achieve a more robust and sustainable approach to late blight management, safeguarding their potato crops and contributing to long-term agricultural sustainability [5][9].
While fungicides have long been the global mainstay for preventing late blight in potato crops, their effectiveness comes with limitations and potential drawbacks [10][56]. Their protective nature is gradually weathering and breaking down over time [13]. Consequently, repeated applications are necessary to safeguard new growth, particularly when disease pressure is high [7]. While timely application can halt or slow symptom development, they cannot ‘cure’ existing infections. This underscores the importance of preventive or early-stage intervention for optimal efficacy [8]. Furthermore, the limited ability of most fungicides to combat established infections within plant tissues highlights a crucial weakness [56]. This is further compounded by the emergence of new, resistant pathogen strains, particularly through mating events in P. infestans. These new strains can render previously effective systemic fungicides useless.
Despite these limitations, fungicide use undeniably contributes to increased potato yields by mitigating late blight’s detrimental effects [15]. However, responsible and strategic application is crucial to minimize potential drawbacks. This includes: (1) Targeted application: Focusing on preventative measures and early intervention during high disease pressure periods [5]. (2) Reduced reliance on systemic fungicides: Exploring alternative fungicide types and diversifying control strategies to slow the development of resistant strains [13]. (3) Adherence to best practices: Following recommended application rates and frequencies to ensure efficacy while minimizing environmental impact [16].
While fungicides like Ridomil MZ 63.5% WP have shown effectiveness in controlling late blight, research highlight the need for strategic application and potential drawbacks [12][66]. Ridomil MZ 63.5% WP, due to its systemic and protectant action, offers superior control compared to other chemicals such as Chlorothalonil, Mancozeb, and Brestan 10. However, even these showed significant improvements over untreated controls [12][66]. Reduced rates of Ridomil application, as explored by [12], can achieve better disease management while considering economic feasibility [8]. In Tunisia, two fungicides, Ridomil Gold® (Metalaxyl) and Copper Nordox® (Cuprous oxide, Cu2O), were tested for their efficacy in reducing hyphal growth of P. infestans. At a concentration of 50 mg/L active ingredient, Ridomil Gold® achieved a 33.96% reduction in hyphal growth compared to Copper Nordox® (54.28%) and the untreated control (100%). Interestingly, applying both fungicides on the lower side of leaflets led to a significant decrease in infection level compared to other application methods like dipping or droplet placement. However, Copper Nordox® demonstrated superior performance in these alternative application methods, achieving infection inhibition of 53.94% and 56.62% through dipping and droplet placement, respectively. These findings suggest that preventive application of low-dose Metalaxyl, as found in Ridomil Gold®, specifically targeting the lower leaf surface, could be an effective strategy for controlling late blight in vitro [48].
Biocontrol
Continuous research and development of alternative approaches, including host resistance and biocontrol methods, are crucial for long-term sustainable disease control in plant production [67]. While in vitro experiments often fail to perfectly translate to field applications, they provide invaluable knowledge about the mechanisms underlying biocontrol. Trichoderma, a well-studied fungal genus, showcases diverse tactics against P. infestans, including coiling around its prey and secreting enzymes, secondary metabolites, and toxins [68][69].It was observed that Trichoderma harzianum and T. asperellum not only reduced late blight symptoms by 40% but also stimulated tomato plant growth by over 30% and 19%, respectively [69]. Pythium oligandrum, an antagonistic oomycete, exhibits both mycoparasitism and secretes cell wall-degrading enzymes during colonization. Interestingly, its mycoparasitic capabilities likely evolved through gene duplication and horizontal gene transfer, enabling it to utilize various fungi and oomycetes for nutrition [51].
Bacteria like Bacillus, Pseudomonas, and Streptomyces also display biocontrol potential. Bacillus species directly antagonize P. infestans, while Pseudomonas species utilize volatile organic compounds (VOCs) like hydrogen cyanide and aldehydes to inhibit its growth. Additionally, some Pseudomonas strains produce cyclic lipopeptides targeting P. infestans zoospores and siderophores competing for iron, hindering its development [44][55]. Despite the wide range of bio-active compounds produced by bacterial and fungal biocontrol agents (BCAs), their in vitro activities are not always translated to real-world settings [54]. For instance, Despite in vitro reports of 40% P. infestans growth reduction were observed through the use of Trichodex®, a commercial T. harzianum product, the product showed no significant effect on late blight in greenhouse or detached leaf assays [70]. Thus, new selection methods are needed, potentially focusing on bio-surfactant and siderophore production as these seem to correlate with in planta effectiveness [71].
Molecular and genomic studies, combined with in vitro assays, offer further insights into BCA biology and modes of action. For example, studies on Pseudomonas reveal specific loci controlling aggressiveness towards P. infestans, suggesting the possibility of engineering hyper-aggressive strains for future use [72]. Additionally, such studies can shed light on the evolutionary history of BCAs, like Pythium‘s acquired hyperparasitism through horizontal gene transfer [44]. In fact, targeting the P. infestans cell wall, composed mainly of β-D-glucans and cellulose, emerges as a key strategy. Many BCAs secrete cell wall-degrading enzymes, and their potent mixtures could be used to select efficient future biocontrol agents [51][57].
This highlights the importance of selecting biocontrol agents (BCAs) adapted to the target environment. Endophytes, or naturally occurring antagonistic microbes from healthy plants within the disease ecosystem, hold promise due to their pre-existing adaptation. This approach was employed to isolate 2800 Bacillus-like and Pseudomonas-like bacteria from potato agroecosystems [44]. Of these, four strains significantly reduced disease symptoms in greenhouse assays, and one (B. subtilis 30B-B6) even validated its efficacy in a small-scale field trial. Sustainable late blight management demands an integrated approach blending various complementary methods [5]. Cultural practices like crop rotation, sanitation, and optimized planting times form the foundation in minimizing disease pressure [4][46]. Resistant cultivars are crucial, significantly reducing fungicide applications due to their moderate or high resistance levels [17][18]. Chemical control, though included, should be judicious and strategic, targeting fungicide sprays only when necessary to maintain disease below economic thresholds. Optimizing application rates, as demonstrated with reduced-rate Ridomil use, helps minimize environmental impact and production costs [15][16]. IDM benefits include reduced fungicide reliance and associated environmental risks, cost-effectiveness through optimized fungicide use and resistance utilization, increased profitability thanks to lower costs and good management practices, and durable disease control due to the multiple tactics employed. Prioritizing cultural practices and resistant varieties within the IDM framework, with judicious fungicide use only when necessary, ensures sustainable, cost-effective, and environmentally responsible disease management for potato production [73][74][75].
References
| 1 | Akino S, Takemoto D, Hosaka K. Phytophthora infestans: A review of past and current studies on potato late blight. J. Gen. Plant Pathol. 2014;80:24-37. DOI |
| 2 | Riolo M, Aloi F, La Spada F, Sciandrello S, Moricca S, Santilli E, Pane A, Cacciola SO. Diversity of Phytophthora communities across different types of mediterranean vegetation in a nature reserve area. Forests. 2020;11(8):853. DOI |
| 3 | Srisawad N, Petchaboon K, Sraphet S, Tappiban P, Triwitayakorn K. Possible reasons affecting different Phytophthora infestans populations in tomato and potato isolates in Thailand. Diversity. 2023;15(11):1121. DOI |
| 4 | Schiffer-Forsyth K, Frederickson Matika D, Hedley PE, Cock PJA, Green S. Phytophthora in horticultural nursery green waste-a risk to plant health. Horticulturae. 2023;9(6):616. DOI |
| 5 | Ivanov AA, Ukladov EO, Golubeva TS. Phytophthora infestans: An overview of methods and attempts to combat late blight. J. Fungi 2021;7:1071. DOI |
| 6 | Stellingwerf JS, Phelan S, Doohan FM, Ortiz V, Griffin D, Bourke A, Hutten RCB, Cooke DEL, Kildea S, Mullins E. Evidence for selection pressure from resistant potato genotypes but not from fungicide application within a clonal Phytophthora infestans population. Plant Pathol. 2018;67:1528-38. DOI |
| 7 | Cohen Y, Rubin AE, Galperin M. Oxathiapiprolin-based fungicides provide enhanced control of tomato late blight induced by mefenoxam-insensitive Phytophthora infestans. PLos One 2018;13:e0204523. DOI |
| 8 | Quds R, Iqbal Z, Arif A, Mahmood R. Mancozeb-induced cytotoxicity in human erythrocytes: Enhanced generation of reactive species, hemoglobin oxidation, diminished antioxidant power, membrane damage and morphological changes. Pestic. Biochem. Physiol. 2023;193:105453. DOI |
| 9 | Abuley IK, Lynott JS, Hansen JG, Cooke DEL, Lees AK. The EU43 genotype of Phytophthora infestans displays resistance to mandipropamid. Plant Pathol. 2023;72:1305-13. DOI |
| 10 | Ben Naim Y, Cohen Y. Replacing Mancozeb with alternative fungicides for the control of late blight in potato. J. Fungi. 2023;9(11):1046. DOI |
| 11 | Blair JE, Coffey MD, Park SY, Geiser DM, Kang S. A multi–locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genet. Biol. 2008;45(3):266-77. DOI |
| 12 | Tsedaley B. Late blight of potato (Phytophthora infestans) biology, economic importance and its management approaches. J. Biol. Agric. Healthc. 2014;4(25):215-25. |
| 13 | Bianchi S, Nottola SA, Torge D, Palmerini MG, Necozione S, Macchiarelli G. Association between female reproductive health and Mancozeb: Systematic review of experimental models. Int. J. Environ. Res. Public Health. 2020;17:2580. DOI |
| 14 | Chen CH, Wang TC, Black L, Sheu ZM, Perez F, Deahl K. Phenotypic and genotypic changes in the Phytophthora infestans population in Taiwan-1991 to 2006. J. Phytopathol. 2009;157:248-55. DOI |
| 15 | Dall’agnol JC, FerriPezzini M, Suarez Uribe N, Joveleviths D. Systemic effects of the pesticide mancozeb-A literature review. Eur. Rev. Med. Pharmacol. Sci. 2021;25:4113-4120. DOI |
| 16 | Najdabbasi N, Mirmajlessi SM, Dewitte K, Mänd M, Landschoot S, Haesaert G. Combination of potassium phosphite and reduced doses of fungicides encourages protection against Phytophthora infestans in potatoes. Agriculture. 2022;12(2):189. DOI |
| 17 | Dufková H, Berka M, Greplová M, Shejbalová Š, Hampejsová R, Luklová M, Domkářová J, Novák J, Kopačka V, Brzobohatý B, Černý M. The omics hunt for novel molecular markers of resistance to Phytophthora infestans. Plants. 2022;11(1):61. DOI |
| 18 | Rogozina EV, Gurina AA, Chalaya NA, Zoteyeva NM, Kuznetsova MA, Beketova MP, Muratova OA, Sokolova EA, Drobyazina PE, Khavkin EE. Diversity of late blight resistance genes in the VIR potato collection. Plants. 2023;12(2):273. DOI |
| 19 | Robertson NF. The challenge of Phytophthora infestans. In: Advances in plant pathology Phytophthora infestans, the cause of late blight of potato Academic Press, London. 1991;7:1–30 |
| 20 | Shaw DS, Fyfe AM, Hibberd PG, Abdel-Sattar MA. Occurrence of the rare A2 mating type of Phytophthora infestans on imported Egyptian potatoes and production of sexual progeny with A1 mating types from the UK. Plant Pathol. 1985;34:552-6. DOI |
| 21 | Kato M, Sato A, Takahashi K. Oospores of Phytophthora infestans found in the experimental field of potato. Ann. Phytopathol. Soc. Japan 1993;59:770 |
| 22 | Govers LL, Man in ‘t Veld WA, Meffert JP, Bouma TJ, van Rijswick PC, Heusinkveld JH, Orth RJ, van Katwijk MM, van der Heide T. Marine Phytophthora species can hamper conservation and restoration of vegetated coastal ecosystems. Proc. Biol. Sci. 2016;283(1837):20160812. DOI |
| 23 | Ho HH. The taxonomy and biology of Phytophthora and Pythium. J. Bacteriol. Mycol. 2018;6(1):40-5. DOI |
| 24 | Gallegly ME, Hong C. Phytophthora identifying species by morphology and DNA fingerprints. USA: APS Press, 2008. |
| 25 | Kroon LP, Bakker FT, Van Den Bosch GB, Bonants PJ, Flier WG. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genet. Biol. 2004;41(8):766-82. DOI |
| 26 | Kroon LP, Brouwer H, de Cock AW, Govers F. The genus Phytophthora anno 2012. Phytopathology. 2012;102(4):348-64. DOI |
| 27 | Peterson PD, Campbell CL, Griffith CS, James E. Teschemacher and the cause and management of potato blight in the United States. Plant Dis. 1992;76:754-6. |
| 28 | Nishimura R, Sato K, Lee WH, Singh UP, Chang T, Suryaningsih E, Suwonakenee S, Lumyong P, Chamswarng C, Tan W, Shrestha SK, Kato M, Fujii N, Akino S, Kondo N, Kobayashi K, Ogoshi A. Distribution of Phytophthora infestans populations in seven Asian countries. Ann. Phytopathol. Soc. Japan 1999;65:163-70. |
| 29 | Patterson DJ. Stramenopiles: Chromophytes from a protistan perspective., Oxford: Clarendon, 1989. |
| 30 | Fry WE, Goodwin SB, Dyer AT, Matuszak JM, Drenth A, Tooley PW, Sujkowski LS, Koh YJ, Cohen BA, Spielman LJ, Deahl KL, Inglis DA, Sandlan KP. Historical and recent migrations of Phytophthora infestans: Chronology, pathways, and implications. Plant Dis. 1993;77:653-61. DOI |
| 31 | Cooke LR, Kildea S, Mehenni-Ciz J, Quinn L, Little G, Hutton F, Perez FM, Deahl KL, Griffin D. Ongoing changes in the Irish potato late blight population. In: PPO-Special Report No. 15, Lelystad, 2012;75-80. |
| 32 | Deahl KL Characterization of Phytophthora infestans populations in North America from the 2009–2011 late blight epidemics. In: PPO-Special Report No.15, Lelystad, 2012;57-8. |
| 33 | Kiiker R, Skrabule I, Ronis A, Cooke DE, Hansen JG, Williams IH, Mänd M, Runno-Paurson E. Diversity of populations of Phytophthora infestans in relation to patterns of potato crop management in Latvia and Lithuania. Plant Pathol. 2019;68:1207-14. DOI |
| 34 | Sedegui M, Carroll RB, Morehart AL, Evans TA, Kim SH, Lakhdar R, Arifi A. Genetic structure of the Phytophthora infestans population in Morocco. Plant Dis. 2000;84:173-6. DOI |
| 35 | Lebreton L, Andrivon D. French isolates of Phytophthora infestans from potato and tomato differ in phenotype and genotype. Eur. J. Plant Pathol. 1998;104:583-94. DOI |
| 36 | Oyarzun PJ, Ordoñes ME, Forbes GA, Fry WE. First report of Phytophthora infestans A2 mating type in Ecuador. Plant Dis. 1997;81:311. DOI |
| 37 | Goodwin SB, Drenth A. Origin of the A2 mating type of Phytophthora infestans outside Mexico. Phytopathology. 1997;87:992-9. DOI |
| 38 | Chycoski CI, Punja ZK. Characteristics of populations of Phytophthora infestans from potato in British Columbia and other regions of Canada during 1993 to 1995. Plant Dis. 1996;80:579-89. DOI |
| 39 | Shattock RC, Shaw DS, Fyfe AM, Dunn JR, Loney KH, Shattock JA. Phenotypes of Phytophthora infestans collected in England and Wales from 1985 to 1988: mating type, response to metalaxyl and isoenzyme analysis. Plant Pathol. 1990;39:242-8. DOI |
| 40 | Guo L, Zhu XQ, Hu CH, Ristaino JB. Genetic structure of Phytophthora infestans populations in China indicates multiple migration events. Phytopathology. 2010;100: 997-1006 DOI |
| 41 | Elansky S, Smirnov A, Dyakov Y, Dolgova A, Filippov A, Kozlovsky B, Kozlovskaya I, Russo P, Smart C, Fry W. Genotypic analysis of Russian isolates of Phytophthora infestans from the Moscow region, Siberia and Far East. J. Phytopathol. 2001;149:605-11. |
| 42 | Lu J, Liu T, Zhang X, Li J, Wang X, Liang X, Xu G, Jing M, Li Z, Hein I, Dou D. Comparison of the distinct, host-specific response of three Solanaceae hosts induced by Phytophthora infestans. Int. J. Mol. Sci. 2021;22(20):11000. DOI |
| 43 | Bouws H, Finckh MR. Effects of strip intercropping of potatoes with non-hosts on late blight severity and tuber yield in organic production. Plant Pathol. 2008;57(5):916-27. DOI |
| 44 | Caulier S, Gillis A, Colau G, Licciardi F, Liépin M, Desoignies N, Modrie P, Legrève A, Mahillon J, Bragard C. Versatile antagonistic activities of soil-borne Bacillus spp. and Pseudomonas spp. against Phytophthora infestans and other potato pathogens. Front. Microbiol. 2018;9:143. DOI |
| 45 | Tiwari JK, Bairwa A, Bhatia N, Zinta R, Kaushal N, Kumar V, Sharma AK, Sharma S, Choudhary B, Luthra SK, Buckseth T. Resistance evaluation for native potato accessions against late blight disease and potato cyst nematodes by molecular markers and phenotypic screening in India. Life. 2023;13(1):33. DOI |
| 46 | Giachero ML, Declerck S, Marquez N. Phytophthora root rot: Importance of the disease, current and novel methods of control. Agronomy. 2022;12(3):610. DOI |
| 47 | Hansen ZR, Everts KL, Fry WE, Gevens AJ, Grünwald NJ, Gugino BK, Johnson DA, Johnson SB, Judelson HS, Knaus BJ, McGrath MT. Genetic variation within clonal lineages of Phytophthora infestans revealed through genotyping-by-sequencing, and implications for late blight epidemiology. PLoS One. 2016;11:e0165690. DOI |
| 48 | Rhouma A, Ben Salem I, Boughalleb-M’Hamdi N, Gomez JIRG. Efficacy of two fungicides for the management of Phytophthora infestans on potato through different applications methods adopted in controlled conditions. Intern. J. Appl. Pure Sci. Agri. 2016;2(12):39-45. |
| 49 | Skelsey P, Rossing WA, Kessel GJT, PowollJ, Van Der Werf W. Influence of host diversity on the development of epidemics. An evaluation and elaboration of mixture theory. Phytopathology. 2005;95(4):328. DOI |
| 50 | Runno-Paurson E, Agho CA, Zoteyeva N, Koppel M, Hansen M, Hallikma T, Cooke DEL, Nassar H, Niinemets Ü. Highly diverse Phytophthora infestanspopulations infecting potato crops in Pskov region, north-west Russia. J. Fungi. 2022;8(5):472. DOI |
| 51 | Liang D, Andersen CB, Vetukuri RR, Dou D, Grenville-Briggs LJ. Horizontal gene transfer and tandem duplication shape the unique CAZyme complement of the mycoparasitic oomycetes Pythium oligandrum and Pythium periplocum. Front. Microbiol. 2020;11:2609. DOI |
| 52 | Gallegly M, Galindo J. Mating types and oospores of Phytophthora infestans in nature in Mexico. Phytopathology. 1958;48:274. |
| 53 | Andrivon D, Lucas JM, Ellisseche D. Development of natural late blight epidemics in pure and mixed plots of potato cultivars with different levels of partial resistance. Plant Pathol. 2003;52:586-94. DOI |
| 54 | Ristaino J. Pioneering Women in Plant Pathology. American Phytopathological Society, APS Press. 2007. |
| 55 | Cray JA, Connor MC, Stevenson A, Houghton JDR, Rangel DEN, Cooke LR, Hallsworth JE. Biocontrol agents promote growth of potato pathogens, depending on environmental conditions. Microb. Biotechnol. 2016;9:330-54. DOI |
| 56 | Khadka RB, Chaulagain B, Subedi S, Marasini M, Rawal R, Pathak N, Sharma-Poudyal D. Evaluation of fungicides to control potato late blight (Phytophthora infestans) in the plains of Nepal. J. Phytopathol. 2020;168:245-53. DOI |
| 57 | Kubicek CP, Steindorff AS, Chenthamara K, Manganiello G, Henrissat B, Zhang J, Cai F, Kopchinskiy AG, Kubicek EM, Kuo A, Baroncelli R, Sarrocco S, Noronha EF, Vannacci G, Shen Q, Grigoriev IV, Druzhinina IS. Evolution and comparative genomics of the most common Trichoderma species. BMC Genom. 2019;20:485. DOI |
| 58 | Enciso-Maldonado GA, Lozoya-Saldaña H, Colinas-Leon MT, Cuevas-Sanchez JA, Sanabria-Velázquez AD, Bamberg J, Raman KV. Assessment of wild Solanum species for resistance to Phytophthora infestans (Mont.) de Bary in the Toluca valley, Mexico. Am. J. Potato Res. 2022;99:25-39. DOI |
| 59 | Beninal L, Bouznad Z, Corbiere R, Belkhiter S, Mabon R, Taoutaou A, Keddad A, Runno-Paurson E, Andrivon D. Distribution of major clonal lineages EU_13_A2, EU_2_A1, and EU_23_A1 of Phytophthora infestans associated with potato late blight across crop seasons and regions in Algeria. Plant Pathol. 2022;71:458-69. DOI |
| 60 | Bhardwaj V, Salej S, Ashwani K, Vanishree G, Sanjeev S, Sundaresha S. Efficiency and reliability of marker assisted selection for resistance to major biotic stresses in potato. Potato J. 2019;46:56-66. |
| 61 | Rhouma A, Mehaoua MS, Mougou I, Rhouma H, Shah KK, Bedjaoui H. Combining melon varieties with chemical fungicides for integrated powdery mildew control in Tunisia. Eur. J. Plant Pathol. 2023;165:189-201. DOI |
| 62 | Kassa B, Sommartya T. Effect of intercropping on potato late blight, Phytophthora infestans (Mont.) de Bary development and potato tuber yield in Ethiopia. Agric. Nat. Resour. 2006;40:914-24. |
| 63 | Tiwari JK, Buckseth T, Zinta R, Bhatia N, Kardile HB, Singh RK, Kumar M. Germplasm, breeding, and genomics in potato improvement of biotic and abiotic stresses tolerance. Front. Plant Sci. 2022;13:805671. DOI |
| 64 | Tiwari JK, Rawat S, Luthra SK, Zinta R, Sahu S, Varshney S, Kumar V, Dalamu D, Mandadi N, Kumar M, Chakrabarti SK. Genome sequence analysis provides insights on genomic variation and late blight resistance genes in potato somatic hybrid (parents and progeny). Mol. Biol. Rep. 2021;48:623-35. DOI |
| 65 | Szajko K, Plich J, Przetakiewicz J, Sołtys-Kalina D, Marczewski W. Comparative proteomic analysis of resistant and susceptible potato cultivars during Synchytrium endobioticum infestation. Planta. 2020;251:4. DOI |
| 66 | Bekele K, Hailu B. Efficacy and economics of fungicide spray in the control of late blight of potato in Ethiopia. Afr. Crop Sci. J. 2001;9:245-50. DOI |
| 67 | Okon OG, Rhouma A, Ismaila U, Matrood AAA, Hajji-Hedfi L. Biological control of fruit rot of postharvest orange (Citrus aurantium) by aqueous plant extracts. Indian J. Agric. Sci. 2023;93(11):1243-7. DOI |
| 68 | De Silva NI, Brooks S, Lumyong S, Hyde KD. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019;33(2):133-48. DOI |
| 69 | Kariuki WG, Mungai NW, Otaye DO, Thuita M, Muema E, Korir H, MassoC. Antagonistic effects of biocontrol agents against Phytophthora infestans and growth stimulation in tomatoes. Afr. Crop Sci. J. 2020;28:55-70. DOI |
| 70 | Stephan D, Schmitt A, Martins Carvalho S, Seddon B, Koch E. Evaluation of biocontrol preparations and plant extracts for the control of Phytophthora infestans on potato leaves. Eur. J. Plant Pathol. 2005;112:235-46. DOI |
| 71 | Bailly A, Weisskopf L. Mining the volatilomes of plant-associated microbiota for new biocontrol solutions. Front. Microbiol. 2017;8:1638. DOI |
| 72 | De Vrieze M, Varadarajan AR, Schneeberger K, Bailly A, Rohr RP, Ahrens CH, Weisskopf L. Linking comparative genomics of nine potato-associated Pseudomonas isolates with their differing biocontrol potential against late blight. Front. Microbiol. 2020;11:857. DOI |
| 73 | Hajji-Hedfi L, Rhouma A, Hajlaoui H, Hajlaoui F, Rebouh NY. Understanding the influence of applying two culture filtrates to control gray mold disease (Botrytis cinerea) in tomato. Agronomy. 2023;13(7):1774. DOI |
| 74 | Hajji-Hedfi L, Hlaoua W, Rhouma A, Al-Judaibi AA, Cobacho Arcos S, Robertson L, Ciordia S, Horrigue-Raouani N, Navas A, Abdel-Azeem AM. Biological and proteomic analysis of a new isolate of the nematophagous fungus Lecanicillium sp. BMC Microbiol. 2023;23:108. DOI |
| 75 | Hajji-Hedfi L, Rhouma A, Al-Judaibi AA, Hajlaoui H, Hajlaoui F and Abdel Azeem AM. Valorization of Capsicum annuum seed extract as an antifungal against Botrytis cinerea. Waste Biomass Valor. 2023;1-15. DOI |
Cite this article:
Rhouma, A., Hajji-Hedfi, L., Atallaoui, K. Potato late blight: the pathogen, the menace, the sustainable control. DYSONA – Life Science, 2024;5(1): 37-51. doi: 10.30493/dls.2024.445326
