Olugbenga D Oloruntola 1*
1, Department of Animal Science, Adekunle Ajasin University, Akungba-Akoko, Nigeria
E-mail:
olugbenga.oloruntola@aaua.edu.ng
Received: 15/06/2021
Acceptance: 14/09/2021
Available Online: 16/09/2021
Published: 01/10/2021

Manuscript link
https://dx.doi.org/10.30493/dls.2021.290718
Abstract
Phytogenics, the plant-derived natural bioactive compounds, are increasingly used in animal feed to induce antibiotic and antioxidative impacts with the ultimate goal of enhancing their productivity and health. Proximate, phytochemical, mineral composition, and antioxidant activity of Anacardium occidentale leaf powder (AOLP) were determined in this study. The proximates: nitrogen-free extract (49.58 %), crude fiber (16.95 %), crude protein (14.65 %), crude fat (10.81 %), and ash (3.70 %) were determined, and bioactive compounds such as tannins, flavonoids, phenols, saponins, alkaloids, and phytate were also quantifiable in AOLP. Zinc (6.65 mg/kg), calcium (5.76 mg/kg), phosphorus (6.18 mg/kg) and magnesium (1.96 mg/kg) were also recorded in AOLP. The Phytate:Zn ratio of AOLP in this study falling above 10 and Calcium:Phytate molar ratio being lower than 6 signaled impaired Zn availability. However, the value of [Ca] x [Phy]: [Zn] of AOLP in this study being less than 0.5 suggests unimpaired Zn bioavailability. The value recorded for 2,2′-diphenyl-1-picrylhydrazyl and vitamin C in this study refers to the antioxidant capacity of AOLP. Therefore, AOLP could be used as a phytogenic supplement in animal feeding models.
Keywords: Cashew, Antioxidants, Bioactive compounds, Dietary supplements
Introduction
The search for an alternative to antibiotics to promote development, feed quality, and prevent infections and diseases in animal production is currently on the rise. Consumers are becoming more aware of the potential damage synthetic antibiotics may cause to public health [1]. For these reasons, the use of phytochemicals as antibiotic alternatives and growth promoters in animal production is becoming more common [2].
Various plant-derived supplements such as clove basil (Ocimum gratissimum) leaf powder, wild mango (Irvingia gabonensis) kernel powder [3], moringa leaf meal, garlic rhizome meal [4], cloves (Syzygium aromaticum) leaf meal, nutmeg (Myristica fragrans) seed meal [5], pawpaw, mustard, black cumin seeds meal [6], Mucuna pruriens leaf, bamboo leaf, neem leaf [7][8] with many other were used in animal production to enhance growth and in some cases improve the animal products’ quality [2][9].
Another promising plant is cashew (Anacardium occidentale L.), which parts or extract may be used in animal production [10]. A. occidentale is a tropical evergreen tree native to Brazil’s northeastern and northern regions and grows to a height of 8 to 15 meters. It is now grown worldwide, especially in Nigeria, India, Vietnam, Benin, Ivory Coast, Philippines, Indonesia, and Guinea-Bissau. The plant belongs to the family Anacardiaceae and the genus Anacardium [11]. Almost all parts of the tree (such as the stem bark and leaves) have been extensively used as traditional herbal therapeutics, benefiting global health [12].
Natural anti-microbials are present in A. occidentale leaf, as they are in many other plants. Secondary metabolites such as anthocyanins, alkaloids, flavonoids, saponin, and tannins have been found in A. occidentale leaves [10][13]. Therefore, A. occidentale leaves fungicidal, anti-microbial [14], and antioxidant activities [12], along with their impressive minerals and vitamin profiles [15], were previously reported. Based on previous researches, it is hypothesized that processing A. occidentale leaf to powder and using it as a supplement in animal feed formulation may provide health, performance, and economic benefits to both the animal and the consumer of animal protein.
The chemical composition of A. occidentale leaf has been extensively studied and reported; however, recent reports indicate that phytochemical or botanical variation exists due to factors such as season, ontogeny, insect herbivory, diurnal rhythm, and abiotic conditions [16]. As a result, regular or periodic examination and characterization of plant parts are needed to determine their effectiveness in producing results when used as a dietary supplement. This study investigates the proximate composition, phytochemical composition, antioxidant activity, and mineral composition of A. occidentale leaf. Additionally, the interrelationships between phytate solubility with calcium and zinc bioavailability in A. occidentale leaf were investigated.
Materials and methods
Plant part collection and processing
Anacardium occidentale leaves were collected in October 2020 at the Adekunke Ajasin University, Akungba Akoko (AAUA) in Nigeria. The leaves were shade-dried for twelve days before being pulverized with a 0.5 mm screen. The resulted A. occidentale leaf powder (AOLP) was stored in plastic rubber in the freezer until needed. Each analysis was conducted thrice.
Anacardium occidentale leaf proximate analysis
AOLP was analyzed for ash, crude fibre, crude fat, crude protein, and nitrogen-free extract using the AOAC method [17].
Phytochemical determination
All reagents and chemicals used for chemical analysis were purchased from Sigma-Aldrich. All of the chemicals used in this experiment were analytical reagent grade.
Tannins
400 g of AOLP was soaked in 2000 ml 70% ethanol, shaken for six hours, then left undisturbed for another 48 hours before filtering through Whatman No 1 filter paper. Using a rotary evaporator, the AOLP ethanolic extract was vacuum condensed at 35-40°C.
200 g of leaf powder was immersed in 1000 ml 70% ethanol, vibrated continuously for six hours before being left undisturbed for another 48 hours and filtered using Whatman No 1 filter paper.
The Folin-Ciocalteau technique [18] was used to determine total tannins. In a volumetric flask (100 ml), 1 ml of the leaf ethanolic extract was diluted with 49 ml distilled water, 1.7 ml 75% ethanol, 0.1 ml metaphosphoric acid, 1.0 mol/ml Na2CO3 (10 ml), and 2.5 ml Folin-Ciocalteu. The mixture was thoroughly blended and left at room temperature for 15 minutes. Following that, the absorbance of standard solution and leaf extract was measured in a spectrophotometer at 680 nm against a blank. As a reference, the standard curve (R2 = 0.9972) was used, and the total tannin content of the sample was expressed as tannic acid (TA) mg TA/g DW.
Flavonoids
Surana et al. [19] method for determining the flavonoids content of leaf samples was followed. In a test tube containing 0.50 ml of leaf powder extract, 0.1ml aluminium chloride solution, 1.50 ml methanol, 0.1 ml potassium acetate solution, and 2.8 ml distilled water were added. Both extract and rutin standard dilutions (10-100 g/ml) sample blanks were made the same way, but with distilled water instead of aluminium chloride solution. After that, the solutions were filtered through Whatman filter paper (No. 1). Absorbance ratios were measured at 510 nm against blanks. Then, total flavonoid content was determined as equivalent to 1 mg rutin per gram of the ethanolic leaf extract.
Phenols
The Folin-Ciocalteau method [20] was used to determine the total phenolic content of the leaf sample. In 50 μL of leaf extract or standard solution, 250 μL of Folin-Ciocalteau reactive was added. This combination was kept at room temperature for 5 minutes in a dark environment. A 750 μL 7 percent Na2CO3 solution was added at the end of this period. Distilled water was used to dilute the mixture to 5 mL. After that, the mixture was kept at room temperature in a dark environment for 120 minutes to react. The absorbance of the samples and standards were measured at 760 nm. Instead of 50 μL extract, an 80 percent methanol solution (50 μL) was added to the blank solution. The total phenolic content was determined using a calibration curve using gallic acid equivalent standards.
Total saponins
The vanillin and concentrated sulfuric acid colourimetric method described in [21] was used for quantifying saponin. The 0.1 ml extract was mixed with 0.5 ml ethanol (50%, w/v), 4.0 ml sulfuric acid (77% w/v), and 0.5 ml of freshly prepared vanillin solution (8% w/v). The mixture was allowed to cool to room temperature before being heated in a water bath to 60 °C for 15 minutes. A UV/Vis spectrophotometer was used to detect the absorbance at 545 nm. The total saponin content in each sample was determined using a tea saponin calibration curve and represented as mg tea saponin equivalent per g (TSE/g DW).
Alkaloids
The gravimetric method [22] was used to determine the alkaloid content of the leaf sample. 5 g of the AOLP was distributed in 50 ml of acetic acid solution in ethanol (10%, w/v). The mixture was subjected to vibration and left undisturbed for about 240 minutes before it was sieved. The filtrate was lessened to a quarter of its initial volume on a hot plate. The alkaloids were then precipitated by adding drops of concentrated ammonium hydroxide. The filter paper was used to filter the precipitate, which was then washed with a 1 percent ammonium hydroxide solution. The precipitate was then oven-dried for half an hour at 60°C before being transferred to desiccators and reweighed until it reached a constant weight. The weight of the alkaloids was calculated as a percentage of the sample weight.
Phytate
The phytate in the leaf samples was quantified using the anion exchange methods described by [23]. The previous AOLP filtrate (0.2-1.0 ml) was diluted with distilled water to a final volume of 1.4 ml, then 1.0 ml ferric ammonium sulfate solution containing 50 µg Fe was added and adequately mixed. The test tubes were then sealed and immersed in a boiling water bath for 20 minutes. Then, 5 ml amyl alcohol was added to the test tube once it had cooled to room temperature, followed by 0.1 ml of ammonium thiocyanate solution (100 g/l). The contents of the test tubes were immediately mixed using inversion and shaking. The colour intensity in the amyl layer was measured with a spectrophotometer at 465 nm against an amyl alcohol blank precisely 15 minutes after ammonium thiocyanate application and brief centrifugation at low speed. Since ferric ions complexed with phytate at pH 1-2 cannot combine with thiocyanate ion to form the characteristic pink complex, the absorbance at 465 nm in the amyl layer is inversely linked to the phytate anion concentration.
Antioxidant activity
DPPH
To measure the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) antioxidant activity of the leaf sample, the DPPH radical degradation activity method [20] was used. The DPPH radical was produced using pure methanol at a concentration of 6×10-5 M to 100µl of sample extract or standard solution, 2µl of methanolic DPPH solution were added. This mixture was kept in the dark for 20 minutes. The sample absorbance was then measured at 515 nm. A blank solution of pure methanol was utilized. Instead of 100 microliters of extract, 100 microliters of pure water were utilized in the control solution. The antioxidant capabilities of sample extracts were determined using a calibration curve produced with varying concentrations (10–100 ppm) of gallic acid solution.
Vitamin C
Vitamin C content of the AOLP was ascertained following the technique mentioned in [24]. 75 μl DNPH solution [i.e., 2 g dinitrophenyl hydrazine, 270 mg copper sulfate (CuSO4. 5H2O) and 230 mg thiourea in 100 ml of 5 ml/L H2SO4] was added to 500 μl extract mixture (300 μl of an adequate dilution of AOLP extract with 100 μl of 13.3% (v/v) trichloroacetic acid and water. The reaction mixture was then incubated at 37 °C for 3 hours before adding 0.5 ml of H2SO4 (65% v/v) to the medium and measuring the absorbance at 520 nm with a UV spectrophotometer. The leaf powder’s vitamin C concentration was then established using ascorbic acid as a reference compound.
Mineral composition
The colourimetric method (modified from AOAC 965.17) was used to determine the amount of phosphorus. In a 100 mL volumetric flask, an aliquot of the leaf sample solution containing 0.2 to 1.5 mg P was placed. Then 20 mL of molybdovanadate reagent was added and stirred thoroughly after being diluted to volume with distilled water After allowing the solution to stand for 10 minutes, the absorbance was read at 400 nm using H2O as the blank. The concentration of P was calculated using the standard curve.
Zn, Ca, and Mg of the leaf sample were analyzed using Atomic Absorption Spectrophotometer (Bulk scientific, USA, model 210 VGP) after wet digestion with a mixture of nitric acid, sulphuric acid, and hydrochloric acid.
Phytate/Zn and phytate/Ca molar ratios
The phytate-to-zinc molar ratio for the AOLP was calculated as follows [25]:
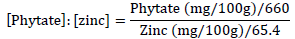
The calcium to phytate molar ratio for the AOLP was also calculated as follows [26]:
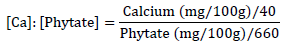
The calcium to phytate:zinc molar ratios was calculated as follows [27]:
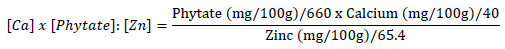
Results and discussion
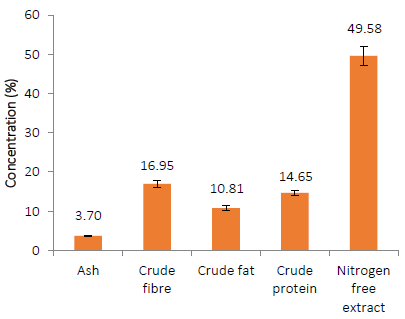
Quantifying the proximates of a common feed ingredient or supplement is crucial for exhibiting its nutritional profile and selecting the proper amount to include in a compounded feed. Nitrogen free extract (49.58 %), crude fibre (16.95 %), crude protein (14.65 %), crude fat (10.81 %), and ash (3.70 %) are all present in A. occidentale leaf powder (Fig. 1). This result is at variance with [28], who reported 54.98%, 3.40%, 0.12%, 24.0%, and 3.5% for nitrogen-free extract, crude fiber, crude protein, crude fat, and ash, respectively in air-dried cashew leaf powder. AOLP proximate composition reveals that it could be a source of energy, protein, and minerals [29][30] [31].
It was anticipated that including secondary metabolite-containing plant tissues in animal meals would be beneficial [32]. The presence of quantifiable tannins, flavonoids, phenols, saponins, alkaloids, and phytate in AOLP (Fig. 2) suggests that when incorporated into the animals’ diet at safe levels, this leaf meal could confer desired or beneficial physiological effects that could improve the animals’ growth and health. The presence of tannin in AOLP corroborated previous findings [33]. Although dietary tannin has been linked to decreases in feed intake, nutritional digestibility, and metabolism in experimental animals [34], it also has some beneficial effects such as lowering blood pressure, modulating immunological response, accelerating blood flow, and lowering serum cholesterol concentrations at tolerable dietary levels [35]. Furthermore, tannins’ anti-allergic, anti-microbial, anti-cancerous, and anti-inflammatory properties have recently been revealed [34].
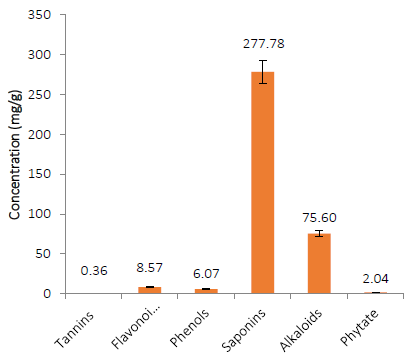
The currently reported levels of flavonoids and phenols in AOLP agree with the findings of [36]. The presence of the aforementioned bioactive compounds in the leaf meal could be of health benefits. For instance, vasorelaxation and antihypertensive effects had been linked to the flavonoid and phenolic compounds [37]. The generation of nitric oxide, the accumulation of cyclic guanosine 3′,5-monophosphate, and endothelium-dependent relaxation in aortic rings have been reported at relatively low polyphenolic compound concentrations (10-5 to 10-2 g/L). In contrast, flavonoids cause vasorelaxation, probably due to the inhibition of cyclic nucleotide phosphodiesterase [38].
Saponins are structurally related chemicals that comprise a steroid or triterpenoid aglycone linked to one or more oligosaccharide moieties. They are responsible for giving a bitter taste and astringency to plant materials due to their hemolytic activity and foaming capabilities [39]. Saponins have anti-cancer properties by affecting the immune system. They also decrease cholesterol and blood sugar levels [40]. It was observed, in this study, that the concentration of saponins in AOLP was relatively high compared to other examined phytochemicals. This content could be beneficial since an optimal dietary saponin content impedes the operative mucosal transport, increases the villi diameter and villi permeability, and consequently enhances the growth performance [41].
In the plant tissues, alkaloids occur as esters (aconitine, atropine, cocaine, scopolamine), water-soluble salts of organic acids (malic, lactic acid, citric, tartaric, acetic, and oxalic), or merge with sugars or tannins rather than as free bases [42]. Alkaloids have the highest concentration of any phytochemicals tested in this study in the AOLP. This could be of health benefits since alkaloids have various pharmacological actions, including anti-cancer, antibacterial, antihyperglycemic, and analgesic properties [43]. Alkaloids also have neuroprotective properties, slowing the progression of neurodegenerative illnesses by acting as an antagonist of N-methyl-D-aspartate (NMDA), boosting gamma-aminobutyric acid levels, and inhibiting the acetyl-cholinesterase enzyme [44][45][46]. Therefore, incorporatingAOLP in animal diets may provide anti-stress qualities.
The result of the phyto-analysis of AOLP shows quantifiable levels of phytate. This could be significant since phytate, as a particularly negatively charged ion, performs a variety of functions in a wide pH range. Thus, its presence in the diet reduces the bioavailability of divalent and trivalent mineral ions [47]. Phytate also forms complexes with proteins, affecting the protein structure and lowering its enzymatic activity, solubility, and proteolytic digestibility [47]. However, dietary phytate cannot be considered entirely unsafe because of its function in preventing atherosclerosis and coronary heart disease, diabetes mellitus, cancer, and kidney stones [47][48]. The phytochemicals concentration of AOLP in this present study differs slightly from 1.19 mg/g, 2.73 mg/g, 0.74 mg/g and 0.57 mg/g reported for tannins, flavonoids, saponins and alkaloids, respectively earlier reported by [49].
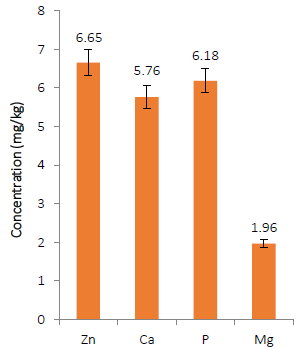
Minerals in animal feed are essential for the metabolic activities of animals. As shown in (Fig. 3), the AOLP has a quantifiable amount of zinc (6.65 mg/kg), calcium (5.76 mg/kg), phosphorus (6.18 mg/kg) and magnesium (1.96 mg/kg). The values reported for Zn, Ca, and P in this study were lower compared to the earlier report of [31]. This information on the mineral composition of AOLP is essential, especially for determining mineral adequacy and dietary intake to acquire data on mineral element composition of feed ingredients or diets [50]. Zinc, one of the minerals found in AOLP in this study, has a wide range of functions, including being a cofactor and constituent of several enzymes (glutamic dehydrogenase, lactate dehydrogenase, alkaline phosphatase, and alcohol dehydrogenase). Additionally, zinc plays key roles in amino acid and nucleic acid metabolism, gene expression, and cell replication, as well as regulating vitamin E and A metabolism and bioavailability, tissue repair, and wound healing [51]. Calcium is an essential dietary mineral because of its functions as a component of teeth and bones, as well as its roles in blood coagulation, muscle and nerve function, enzyme activation (e.g. succinic dehydrogenase, adenosine triphosphatase, lipase), membrane permeability, and nerve impulse transmission [52]. Phosphorus plays an important role in a variety of metabolic processes and is found in phosphorylated metabolic intermediates, teeth, bones, adenosine triphosphate, and nucleic acids [51]. Magnesium is an essential mineral that acts as an activator for the enzymes diphophopyridinenucleotide kinase, creatine kinase, and phosphate-transferring enzymes myokinase. Magnesium also activates the enzymes pyruvic acid oxidase and carboxylase [52].
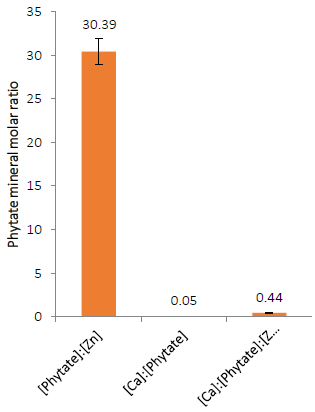
The Phytate: Zinc, Calcium: Phytate and [Calcium] x [Phytate]: [Zinc] molar ratios are depicted in (Fig. 4). Feed with Phytate: Zn molar ratio of less than 10 are considered to have adequate Zn availability [53][54]. The Phytate:Zn of AOLP in this study falling above this value suggests impairment of Zn availability. Under another model, the level of Ca in the intestine influences the solubility of phytate and the proportion of Zn bound in a mineral complex. Furthermore, total phytate precipitation takes place when Ca:Phy molar ratio reaches an approximate level of 6:1 [55][56]. Therefore, the current Calcium:Phytate molar ratio lower than the 6 in this study also suggest an impaired Zn availability under this model. However, in another reportedly better model i.e. [Ca] x [Phytate]: [Zn], the Zn availability is considered impaired when the value for [Ca] x [Phytate]: [Zn] is higher than 0.5 [57][58]. Therefore the value of [Ca] x [Phy]: [Zn] of AOLP in this study being less than 0.5 suggests unimpaired Zn bioavailability.
The antioxidant activity of AOLP powder is shown in (Fig. 5). The antioxidant qualities of medicinal plants improve the protection they provide against disease. Consumption of phytogenic antioxidants has been proven to be inversely related to morbidity and death from degenerative diseases [59]. The antioxidant capacity reported in the current study (42.24%) suggests that the phytoconstituents of AOLP can donate hydrogen to a free radical, hence preventing possible oxidation harm [60].
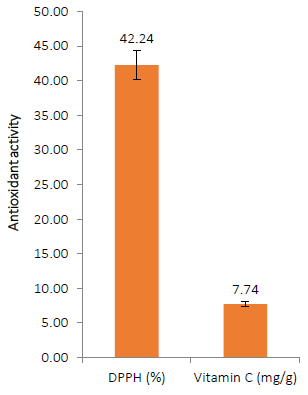
Vitamin C is described as a complex redox network containing enzymes and metabolites with combined effects and reciprocal interactions, and it is an essential component of plant antioxidant systems [61]. The quantifiable amounts of vitamin C (7.72 mg/g) also suggest that using AOLP either as a supplement or component could promote health status [62].
Conclusion
The current study shows that AOLP is high in fibre, protein, energy, and pharmacologically important phytochemicals. The leaf powder has antioxidant capabilities and is a source of Ca, Zn, P, and Mg. However, further studies are needed to evaluate the bioavailability of Ca and Zn in AOLP. Leaf powder from A. occidentale could be used as a phytogenic supplement. Therefore, it is recommended that the leaf powder be used as a supplement in feeding trials with experimental animals.
References
| 1 | Gadde U, Kim WH, Oh ST, Lillehoj HS. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim Health Res Rev. 2017; 18:26–45 DOI |
| 2 | Valenzuela-Grijalva NV, Pinelli-Saavedra A, Muhlia-Almazan A, Dominguez-Diaz D, Gonzalez-Rios H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J Anim Sci Technol. 2017;59(1). DOI |
| 3 | Oloruntola OD, Adu OA, Gbore FA, Falowo AB, Olarotimi OJ. Performance of broiler chicken fed diets supplemented with Irvingia gabonensis kernel powder and Ocimum gratissimum leaf powder. Slovakia J Anim Sci. 2021;54(1):7-20. |
| 4 | Gbore FA, Oloruntola OD, Adu OA, Olarotimi OJ, Falowo AB, Afolayan EO. Serum and meat antioxidative status of broiler chickens fed diets supplemented with garlic rhizome meal, moringa leaf meal and their composite. Trop Anim Health Prod. 2017;53. DOI |
| 5 | Adu OA, Gbore FA, Oloruntola OD, Falowo AB and Olarotimi OJ. The effect of Myristica fragrans seed meal and Syzygium aromaticum leaf meal dietary supplementation on growth performance and oxidative status of broiler chicken. Bull Natl Res Cent. 2020;44. DOI |
| 6 | Adegbeye MJ, Oloruntola OD, Asaniyan EK, Agunbiade B, Oisagah EA, Ayodele SO. Pawpaw, black cumin, and mustard seed meal dietary supplementation in broiler chickens: Effect on performance, gut microflora, and gut morphology. J Agric Sci Tech. 2020;22(5):1235-46. |
| 7 | Oloruntola OD, Agbede JO, Ayodele SO, Oloruntola DA. Neem, pawpaw, and bamboo leaf meal dietary supplementation in broiler chickens: Effect on performance and health status. J Food Biochem. 2018;43(2). DOI |
| 8 | Oloruntola OD, Agbede JO, Ayodele SO, Adeyeye SA, Agbede JO. Performance, haemato-biochemical indices, and antioxidant status of growing rabbits fed on diets supplemented with Mucuna pruriens leaf meal. World Rabbit Sci. 2018;26:277-85. DOI |
| 9 | Lillehoj H, Liu Y, Calsamiglia S, Fernandez-Miyakawa ME, Chi F, Cravens RL, Oh S, Gay CG (2018). Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet Res. 2018;49. DOI |
| 10 | Setiawan H, Jingga ME, Saragih HT. The effect of cashew leaf extract on small intestine morphology and growth performance of Jawa Super chicken. Vet World. 2018;11(8): 1047-54. DOI |
| 11 | De Brito ED, Silva ED, Sueli Rodrigues S. Caju-Anacardium occidentale. In: Exotic Fruits. Academic Press. 2018:85-9. DOI |
| 12 | Salehi B, Gültekin-Özgüven M, Kirkin C, Özçelik B, Morais-Braga M, Carneiro J, Bezerra C F, da Silva, TG, Coutinho H, Amina B, Armstrong L, Selamoglu Z, Sevindik M, Yousaf Z, Sharifi-Rad J, Muddathir AM, Devkota H P, Martorell M, Jugran AK, Cho WC, Martins N. Antioxidant, Anti-microbial, and Anticancer Effects of Anacardium Plants: An Ethnopharmacological Perspective. Front Endocrinol. 2020;11. DOI |
| 13 | Ajileye OO, Oboutor E.M, Akinkunmi EO, Aderogba MA. Isolation and characterisation of antioxidant and anti-microbial compounds from Anacardium occidentale L. (Anacardiaceae) leaf extract. J King Saud Univ. Sci. 2015;27:244-52. DOI |
| 14 | Anand G, Ravinanthan M, Basaviah R, Shetty AV. In vitro anti-microbial and cytotoxic effects of A. occidentale and Mangifera indica in oral care. J Pharm Bioallied Sci. 2015;7(1): 69-74. DOI |
| 15 | Eliakim-Ikechukwu, C, Obri A, Akpa O. Phytochemical and micronutrient composition of A. occidentale Linn (cashew) stem-bark hydroethanolic extract and its effect on the fasting blood glucose levels and body weight of diabetic Wistar rats. Int J Nutr Wellness. 2010;10. |
| 16 | Lämke JS, Unsicker SB. Phytochemical variation in treetops: causes and consequences for tree-insect herbivore interactions. Oecologia. 2018; 187:377–88. DOI |
| 17 | AOAC. Official Methods of Analysis of Association of Offical Analytical Chemists. 18th Edition, Washington DC. 2010. |
| 18 | Biswas A, Dey S, Li D, Yiu L, Zhang J, Huang S, Pan G, Deng Y. Comparison of Phytochemical Profile, Mineral Content, and In Vitro Antioxidant Activities of Corchorus capsularis and Corchorus olitorius Leaf Extracts from Different Populations. J Food Qual. 2020;2020(9). DOI |
| 19 | Surana AR, Kumbhare MR, Wagh RD. Estimation of total phenolic and total flavonoid content and assessment of in vitro antioxidant activity of extracts of Hamelia patens Jacq. stems. Res. J. Phytochem. 2016;10(2):67-74. |
| 20 | Otles S, Yalcin B (2012). Phenolic Compounds Analysis of Root, Stalk, and Leaves of Nettle. Sci World J. 2012; 2012. DOI |
| 21 | He J, Wu ZY, Zhang S, Zhou Y, Zhao F, Peng ZQ, Hu ZW. Optimisation of microwave‐assisted extraction of tea saponin and its application on cleaning of historic silks. J Surfactants Deterg. 2014;17(5):919-28. DOI |
| 22 | Adeniyi SA, Orjiekwe CL, Ehiagbonare JE. Determination of alkaloids and oxalates in some selected food samples in Nigeria. Afr J Biotechnol. 2009; 8(1):110-2. |
| 23 | Davies NT, Reid H. An evaluation of the phytate, zinc, copper, iron and manganese contents of, and zn availability from, soya-based textured-vegetable-protein meat-substitutes or meat-extenders. Br J Nutr. 1979;41(3):579-89. DOI |
| 24 | Benderitter M, Maupoli V, Vergely C, Dalloz F, Briot F, Rochette L. Studies by electron paramagnetic resonance of the importance of iron in the hydroxyl scavenging properties of ascorbic acid in plasma: Effects of iron chelators. Fundam Clin Pharmacol. 1998; 12 (5):510-6. DOI |
| 25 | Amirabdollahian F, Ash R. An estimate of phytate intake and molar ratio of phytate to zinc in the diet of the people in the United Kingdom. Public Health Nutr. 2010;13(9):1380-88. DOI |
| 26 | Norhaizan ME, Nor Faizadatul Ain AW. Determination of phytate, iron, zinc, calcium contents and their molar ratios in commonly consumed raw and prepared food in Malaysia. Mal J Nutr. 2009;15(2): 213-22. |
| 27 | Igwe CU, Ibegbulem CO, Nwaogu LA, Ujowundu CO, Okwu GN. Calcium, zinc and phytate interrelationships in four lesser-known African deeds processed into food condiments. J Adv Chem. 2013;4(1):288-94. |
| 28 | Obayuwana O, Imafidon KE, Abu OD. Phytochemical and proximate composition of leaves of Anacardicum occidental. J Agric Res Life Sci. 2020;5:139-42. |
| 29 | Martínez Y, Martínez O, Olmos E, Siza S, Betancur C. Nutraceutical effect of A. occidentale in diets of replacement laying pullets. Cordoba MVZ J. 2012;17(3):3125-32. |
| 30 | Martínez Y, Escalona A, Martínez O, Olmo C, Rodríguez R, Isert M, Betancur C, Valdivie M and Liu G. The use of A. occidentale as nutraceutical in hypoprotein diets for laying hens. Cuban J Agric Sci. 2012;46(4):395-401. |
| 31 | Martinez Y, Tobar LA, Lagos HM, Parrado CA, Urquia AM and Valdivie M. Phytobiotic effect of A. occidentale L. leaves powder on performance, carcass traits and intestinal characteristics in broilers. Brazilian J Poult Sci. 2021;23(1). DOI |
| 32 | Villalba J, Costes-Thiré M, Ginane C. Phytochemicals in animal health: Diet selection and trade-offs between costs and benefits. Proceed Nutr Soc. 2017;76(2):113-21. DOI |
| 33 | Jaiswal Y, Naik V, Tatke P, Gabhe S, Vaidya A. Pharmacognostic and preliminary phytochemical investigations of Anarcardium occidentale (Lin) leaves. Int J Pharm Pharmaceutl Sci. 2021;4(3):625-31. |
| 34 | Sharma K, Kumar V, Kaur J, Tanwar B, Goyal A, Sharma R, Gat S, Kumar A. Health effects, sources, utilisation and safety of tannins: a critical review. Toxin Rev. 2019. DOI |
| 35 | Chung KT, Wong TY, Wei CI, Huang YW, Lin Y. Tannins and human health: a review. Crit Rev Food Sci Nutr. 1998;38(6):421-64. DOI |
| 36 | Nugroho AE, Malik A, Pramono S. Total phenolic and flavonoid contents and in vitro antihypertension activity of purified extract of Indonesian cashew leaves (A. occidentale L.). Int Food Res J. 2013;20(1):299-305. |
| 37 | Torres-Piedra M, Figueroa M, Hernandez-Abreu O, Ibarra-Barajas M, Navarrete-Varquez G, EstradaSoto S. Vasorelaxant effect of flavonoids through calmodulin inhibitor: ex vivo, in vitro, and in silico approaches. BioorgMed Chem. 2011;19:542-6. DOI |
| 38 | Andriambeloson E, Magnier C, Haan-Archipoff G, Lobstein A, Anton R, Beretz A, Stoclet JC, Andriantsitohaina R. Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J Nutr. 1998;128(12):2324-33. DOI |
| 39 | Mohan VR, Tresina PS, Daffodil ED. Antinutritional factors in legume seeds: Characteristics and determination. In: Encyclopedia of Food and Health. Academic Press. 2016:211-20, DOI |
| 40 | Shi J, Arunasalam K, Yeung D, Kakuda Y, Mittal G, Jiang Y. Saponins from edible legumes: chemistry, processing, and health benefits. J Med Food. 2004;7(1):67-78. DOI |
| 41 | Chaudhary SK, Rokade JJ, Aderao GN, Singh A, Gopi M, Mishra A, Raje K. Saponin in poultry and monogastric animals: A Review. Int J Curr Microbiol Appl Sci. 2018;7(7): 3218-25. DOI |
| 42 | Kukula-Koch WA, Widelski J. Alkaloids. In: Pharmacognosy, Academic Press. 2017:163-198, DOI |
| 43 | Hussain G, Rasul A, Anwar H, Aziz N, Razzaq A, Wei W, Ali M, Li J, Li X. Role of Plant-derived alkaloids and their Mechanism in Neurodegenerative Disorders. Int J Biol Sci. 2018; 14(3):341-57. DOI |
| 44 | Cushnie TP, Cushnie B, Lamb AJ.Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int J Antimicrob Agents. 2014;44(5):377-86. DOI |
| 45 | Cortes N, Posada-Duque RA, Alvarez R, Alzate F, Berkov S, Cardona-Gómez GP, Osorio E.Neuroprotective activity and acetylcholinesterase inhibition of five Amaryllidaceae species: a comparative study. Life Sci. 2015;122:42-50. DOI |
| 46 | Abhijit Dey AM. Plant-Derived Alkaloids: A Promising Window for Neuroprotective Drug Discovery. In: Discovery and Development of Neuroprotective Agents from Natural Products. 2017:237–320. DOI |
| 47 | Gemede HD. Potential health benefits and adverse effects associated with phytate in foods: A review. Global J Med Res: K Interdiscip. 2014;14(3):23-31. |
| 48 | Grases F, March JG, Prieto RM, Simonet BM, Costa-Bauzá A, García-Raja A, Conte A. Urinary phytate in calcium oxalate stones formers and healthy people. Scand J Urol Nephrol. 2000; 34: 162-4. DOI |
| 49 | Onuh JO, Idoko G, Yusufu P, Ohun F. Comparative studies of the phytochemical, antioxidant and antimicrobial properties of cashew leaf, bark and fruits extracts. American J Food Nutr. 5(4): 115-20. DOI |
| 50 | Simsek A, Aykut O. Evaluation of the microelement profile of Turkish hazelnut (Corylus avellana L) varieties for human nutrition and health. Int. J. Food Sci. Nutr. 2007;58: 677-88. DOI |
| 51 | Murray RK, Granner DK, Mayes PA, Rodwell VW. Harper’s Biochemistry, 25th Edition, McGraw-Hill, Health Profession Division, USA. 2000. |
| 52 | Soetan KO, Olaiya CO, Oyewole OE. The importance of mineral elements for humans, domestic animals and plants: A review. African J Food Sci. 2010;4(5):200-2. DOI |
| 53 | Oberleas D, Harland BF. Phytate content of foods: effect on dietary zinc bioavailability. J Am Diet Assoc. 1981;79:433- 36. |
| 54 | Adeyeye EI. Proximate, mineral and antinutrient composition of dika nut (Irvingia gabonensis) kernel. Elixir Int J. 2013; 58: 14902-6. |
| 55 | Wise A. Dietary factors determining the biological activities of phytate. In: Nutrition Abstracts and Reviews (series A). 1983;53:791-806. |
| 56 | Oloruntola OD, Agbede JO, Onibi GE, Igbasan SO, Ayodele SO. Chemical characterisation, energy, and zinc bio-availability of cassava starch residues fermented with rumen liquor and different N-sources. Anim Res Int. 2017;14(3):2842-59. |
| 57 | Ellis R, Kelsay JL, Reynolds RD, Morris ER, Moser PB, Frazier CW. Phytate: zinc and phytate x calcium: zinc millimolar ratios in self-selected diets of Americans, Asian Indians and Nepalese. J Am Diet Assoc.1987;87:1043-47. |
| 58 | Oloruntola OD, Agbede JO, Onibi GE, Igbasan FA. Composition of cassava (Manihot spp.) peels fermented with bovine rumen liquor and different nitrogen sources.J Global Agric Ecol. 2015;2(1): 26-35. |
| 59 | Gulcin I. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012;86:345-91. DOI |
| 60 | Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12. DOI |
| 61 | Paciolla C, Paradiso A, de Pinto MC. Cellular redox homeostasis as central modulator in plant stress. In Redox State as a Central Regulator of Plant-Cell Stress Responses. Springer, Cham, Switzerland. 2016:1–23. DOI |
| 62 | Slavin JL, Lloyd, B. Health benefits of fruits and vegetables. Advan Nutrit. 2012;3(4):506–16. DOI |
Cite this article:
Oloruntola, O. Proximate, phytochemical, mineral composition and antioxidant activity of Anacardium occidentale L. leaf powder. DYSONA – Life Science, 2021;2(4):39-49. doi: 10.30493/dls.2021.290718
