Monica O. Oguntimehin 1, 2*; Adebanjo A. Badejo 2; Victor N. Enujiugha 2
1, Department of Biological Science, Olusegun Agagu University of Science and Technology, Okitipupa, Ondo State, Nigeria
2, Department of Food Science and Technology, Federal University of Technology, Akure, Nigeria
E-mail:
monicaoluwatoyin@gmail.com
Received: 09/05/2022
Acceptance: 04/06/2022
Available Online: 06/06/2022
Published: 01/07/2022

Manuscript link
http://dx.doi.org/10.30493/DAS.2022.339707
Abstract
African star apple (Chrysophyllum albidum) is considered a rich source of bioactive and nutritive chemicals, including flavonoids, phenolics, and carotenoids. This research was conducted to explore the effect of salicylic acid treatment on sugar levels in addition to non-enzymatic and enzymatic antioxidants activities of Chrysophyllum albidum fruits during ambient storage. For this purpose, ripe, healthy fruits of C. albidum were treated with varying doses of salicylic acid (0.1 mM, 0.2 mM, 0.4 mM) for 5, 10, and 15 min at each concentration. The treated fruits were kept at 28 ± 2 °C and a relative humidity of 90 ± 5% for 15 days. The total flavonoids, total phenols, and ascorbic acid of salicylic acid-treated fruits increased considerably compared to untreated fruits after the storage period. When compared with other treated samples, the untreated control samples exhibited the highest overall sugar content, non-reducing sugar content, reducing sugar content, and catalase activity. On the other hand, 0.4 mM salicylic acid treatment for 15 minutes resulted in the highest phenol, flavonoid, and ascorbic acid contents, while 0.4 mM salicylic acid treatment for 15 minutes resulted in the highest value of carotenoids content. Furthermore, salicylic acid treatments preserved ascorbate peroxidase and superoxide dismutase activities. The results showed that 0.2 and 0.4 mM salicylic acid treatments for 15 minutes were the most effective in retaining the investigated fruit biochemical features.
Keywords: Chrysophyllum albidum, African star apple, Salicylic acid, Storage, Antioxidants
Introduction
African star apple (ASA) with the botanical name Chrysophyllum albidum, Linn is an indigenous plant belonging to the Sapotaceae family and is extensively found in Africa (Nigeria, Niger, Uganda, Cote d’Ivoire, and Cameroon). Local names for it in Nigeria’s South-West and South-East regions are “Agbalumo” and “Udara” respectively [1]. It produces an edible large berry fruit with up to five flat-shaped seeds. When in season, it is characterized by a light pinkish yellow endocarp and is enjoyed by all ages. Every year, from December through April, the completely ripe fruit is accessible [2]. Indigenous fruit trees like ASA provide people with valuable fruits contributing to their food security and wellbeing, particularly during times of drought and famine [2].
ASA fruit has been shown to contain a high ascorbic acid concentration estimated to be a hundred times higher than oranges and ten times higher than guava or cashew [3]. More importantly, the fruit is eaten fresh and used to make jelly, jam, stewed fruit, various soft drinks, marmalade, and syrup [4], making it a valuable source of vitamin C in daily diet. Furthermore, ASA contains a potent natural antioxidant used to manage several oxidative stress illnesses involving free radicals [5]. Therefore, the production of ASA fruit has expanded due to its medicinal and culinary qualities. Thus, developing efficient storage and marketing workflows for this fruit is crucial to limiting postharvest loss and maintaining quality since a substantial quantity of its production in Nigeria is lost or discarded due to inadequate management.
Pericarp browning, desiccation, quality loss, postharvest decays, and bio-deterioration are essential issues influencing the storage quality of ASA fruits. Rapid desiccation of fruit causes browning of the pericarp, which reduces consumer appeal and marketability, although the nutritional quality and flavor are retained [6]. The traditional methods of fruit handling, the lack of preservation technology, and the lack of marketing understanding are among the causes of these losses. Simple postharvest quality management measures must be developed to prevent postharvest losses [6].
The use of environmentally friendly technologies in postharvest is highly recommended. For instance, salicylic acid (SA), which is a natural plant compound classed as “GRAS” (generally recognized as safe), can improve the natural resistance to postharvest stresses in stored fruits and vegetables while maintaining the nutritional and sensory qualities, thereby extending postharvest life [7]. Because of its impact on plant physiological processes, SA is identified as a plant growth regulator [8]. It has been reported to postpone the senescence of fruits and inhibit fruit ripening processes during postharvest storage of tomatoes [9]. It was also reported that treatment with salicylates after harvest increased the storage life of grapes, kiwifruit, strawberry, Chinese water chestnut, peach, mandarin, pomegranate, and sweet cherry fruits [10][11]. There are currently no investigations on the postharvest preservation of African star apple fruits using salicylic acid as a therapeutic agent. Consequently, this research intends to examine the effects of salicylic acid treatment on some biochemical attributes (sugar content and antioxidative potentials) of African star apple after ambient storage.
Material and Methods
Materials
Chemicals
All analytical grade chemicals and reagents were obtained from Sigma Aldrich (St. Louis, MO), Merck (England), Avondale labs Banbury, Oxon, England, and Eagle scientific Ltd. Nottingham, NG9 6DZ, England. Glass pure water was utilized, and all glassware was acid cleaned and rinsed with distilled water prior to use.
Fruit samples preparation
For this study, fresh, mature, and healthy African star apple fruits were obtained in the early morning from a local farm and transferred to the Federal University of Technology in Akure, Nigeria. The fruits were sorted and inspected upon arrival at the laboratory to ensure that only healthy fruits were chosen for the study. The fruit was chosen for its homogeneous size, color, hardness, and lack of deterioration [12]. The selected fruits were surface-sterilized for 10 minutes with sodium hypochlorite (500 ppm) and air-dried to avoid microbial contamination.
The fruits were identified and certified by the Botany Department, University of Ibadan, Oyo State, Nigeria, and given the voucher registration number UIH/20I6/22502.
Salicylic acid treatments
For SA treatments, the approach described by [9] was used with a few changes. The samples were arranged into groups of 40 fruits.
Fruit treatments were administered as follows:
UT0: Untreated fruits served as a negative control, DWT: Fruits dipped in distilled water for five, ten, and fifteen minutes served as a positive control. The remaining fruits were dipped in different concentrations of salicylic acid (SA), 0.1, 0.2, and 0.4 mM SA at different dipping times of 5, 10, and 15 minutes at each concentration.
Storage of fruits
All treated fruits were air-dried at room temperature for one hour before being transferred to 10 L sealed and sterile high-density polyethylene (HDPE) containers and stored for 15 days at ambient temperatures (28±2 °C) and a relative humidity of 90±5% . The biochemical attributes of the fruit were evaluated on day zero (at harvest) and the fifteenth day of storage [12].
Fruit extract preparation
Fruits were selected randomly from the control and the treated samples. 50 g from each selected fruit was homogenized in Fisons Scientific equipment whirl mixer with the addition of 50 ml distilled water to get a uniform consistency. The slurry was poured into a measuring cylinder, and the volume was made up to 100 ml using distilled water. The slurry samples were centrifuged using Eppendorf AG centrifuge 5418R for 10 min. The supernatant was stored in a refrigerator at 0 °C as fruit extract for further analysis (whenever mentioned afterward as extract).
Determination of sugar content
The colorimetric Anthrone technique described in [13] was employed to analyze the total sugar determination in fruit juice. Fruit from each treatment was randomly selected and cut into small pieces. The samples were treated with 80% alcohol, and the starch present was extracted using 52% perchloric acid. Then, 5 ml of concentrated sulfuric acid and 4 ml anthrone reagent were added to 0.2 g of the sample. The samples were then heated until the reaction was completed. Sugars in the sample react with the anthrone reagent to generate a blue-green color in acidic conditions. After cooling, the solution absorbance was measured at 630 nm (721G visible spectrophotometer, Searchtech instruments). A calibration curve was created using a set of carbohydrate standards with known concentrations. The reducing sugar content was measured by weighing 10 g of the sample into a 500 ml volumetric flask. Then, water (100 ml) was added and neutralized with NaOH solution to the phenolphthalein endpoint. 10 ml neutral lead acetate solution was added, shaken, and allowed to stand for 10 min. Potassium oxalate solution was added in small quantities until there was no further precipitation. After mixing, the solution was filtered using Whatman filter circle No. 1. Then, 50 ml of the filtrate was pipetted into a volumetric flask. 5 ml of concentrated HCl was added and the mixture was kept at room temperature for 24 hours. Concentrated NaOH followed by 0.1 N NaOH were used for neutralization using phenolphthalein as an end point indicator. The solution was made up to volume and transferred to a 50 ml burette having an off-set tip. The titration was performed against Fehling’s solution to determine the amount of unknown C. albidum pulp sugar solution necessary to completely reduce the standard Fehling’s solution (mixture of 5 ml each of stock solutions A and B). The non-reducing sugar (sucrose) content was estimated by subtracting reducing sugar from total sugar content.
Determination of non-enzymatic antioxidant contents
Determination of phenolic content
The total phenolic content was calculated using the technique described in [14]. 2.5 ml of 10 percent Folin–reagent Ciocalteau’s (vv) was combined with appropriate dilutions of the extracts and neutralized with 2.0 ml of 7.5 percent sodium carbonate. This mixture was incubated at 45 °C for 40 minutes. Then, the mixture absorbance at 765 nm was measured using a spectrophotometer (General scientific. GS- UV32PCS). After serial dilution, the total phenolic content was determined using Gallic acid as a reference (phenol standard stock: 20 mg/ml gallic acid). The total phenolic content of all samples was determined using the following formula [15]:
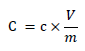
Where,
C: Total phenolic content (mg/g) in gallic acid equivalent (GAE)
C: Gallic acid concentration as obtained from calibration curve (mg/mL)
V: The used fruit extract volume (ml)
m: Extract mass (g).
Determination of total flavonoid content
The fruit extract’s total flavonoid content was assessed using the technique described by [16] with slight modifications. 0.5 mL of properly diluted extract was combined with 0.5 mL of methanol, 50 mL of 10% aluminum chloride (AlCl3), 50 mL of 1 mol/l potassium acetate, and 1.4 mL of water and incubated at room temperature for 30 minutes. The absorbance of the reaction mixture was then measured at 415 nm using a spectrophotometer (JENWAY 6305). The total flavonoid was determined using quercetin stock (5 mg/ml) as a reference and serial dilution. The total flavonoid content was determined as follows:
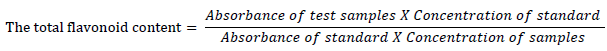
The concentration of total flavonoid content in the test samples was calculated from the calibration plot (𝑌 = 0.0162𝑥 + 0.0044, 𝑅2 = 0.998). The results obtained were expressed as mgQE/g. (QE – Quercetin equivalent).
Ascorbic acid (vitamin C) assay
The technique of [17] was used to determine the vitamin C content in the aqueous extract. 75 μl of DNPH solution (2 g dinitrophenyl hydrazine, 230 mg thiourea, and 270 mg copper sulfate (CuSO4.5H2O) in 100 ml of 5 ml/L H2SO4) was added to a combination of 300 μl extract combined with 100 μl trichloroacetic acid (TCA) (13.3%) and 100 μl water. This mixture was then incubated for 3 hours at 37 °C before adding 0.5 ml of H2SO4 (65% v/v) to the medium. The mixture absorbance at 520 nm was then measured using a UV spectrophotometer (General scientific. GS- UV32PCS). The vitamin C concentration of the extracts was then determined using ascorbic acid as a reference. The following equation is how the results were computed:
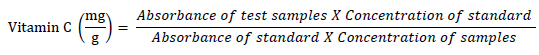
Total carotenoids content
The total carotenoid content of the sample was determined using an acetone-petroleum ether extraction followed by spectrophotometric analysis using the modified technique described by [18]. Ten African star apple fruit (samples) were selected randomly from the control set and the treated samples, the seeds were removed and the pulp was cut into small pieces. Extraction of carotenoid was performed repeatedly by the extraction of 10 g fruit pulp with acetone using pestle and mortar until the sample was colorless. Acetone was then pooled, extracted and transferred to a separating funnel, which contained about 20 ml petroleum ether, and mixed gently. About 20 ml of sodium sulfate solution (5%) was then added and gently mixed well in the separating funnel until the two separate layers were formed. The lower aqueous phase was separated and re-extracted with an additional 20 ml of petroleum ether to remove the color completely. A small amount of distilled water was then used to wash the pooled petroleum ether extract. The carotenoids containing washed petroleum ether extract was poured into a brown bottle with 10 g of anhydrous sodium sulfate. The extract was kept aside for about 30 minutes. Then the extract was decanted into a 100 ml volumetric flask through a funnel fitted with cotton wool. Sodium sulfate slurry was washed with petroleum ether until it was colorless and the washing was transferred into the volumetric flask. The concentration of total carotenoids was measured in a spectrophotometer (General scientific. GS- UV32PCS) using petroleum ether as a blank, it was measured at the absorbance in spectrophotometer at 503 nm [18][19]. The results were given in milligrams per kilogram of fresh weight (FW) (mg/kg FW) [19].
Determination of enzymatic activities
Catalase (CAT) activity
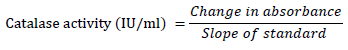
Catalase activity was determined according to the method described by [19][20]. Fruit homogenate (70 μl) was mixed with 920 μl Na-PO4 buffer pH 7 containing 0.1 mM Ethylene diamine tetra acetic acid (EDTA). The reaction was initiated by adding ten μl of H2O2 (30 mM). Then the decrease in H2O2 concentration was measured as the absorbance at 240 nm every 10 seconds for 3 minutes.
Therefore,
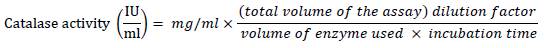
Superoxide dismutase (SOD) activity
The activity of superoxide dismutase (SOD) in the fruit homogenates was determined using the method described in [19][21]. 1 ml of the fruit homogenates was diluted with 9 ml of distilled water to make a 1 in 10 dilution. 1 ml aliquot of the diluted homogenate was added to 2.5 ml of 0.05 M carbonate buffer to equilibrate the spectrophotometer (pH 10.2). The reaction was initiated by inverting 0.3 ml of freshly generated 0.3 mM adrenaline into the liquid, which was quickly stirred. 2.5 ml buffer, 0.3 ml substrate (adrenaline), and 0.2 mL water were in the reference cuvette. Then, the increase in absorbance at 480 nm between the 30 and 150 seconds mark was measured.
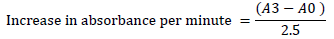
Where, A0 = absorbance after 30 seconds and A3 = absorbance after 150 seconds.
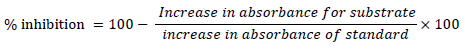
One unit of SOD activity was defined as the amount of SOD necessary to inhibit the oxidation of 50% adrenaline to adrenochrome during 1 minute.
Ascorbate peroxidase (APX) assay
APX activity was measured using the procedure reported in [22][23]. The activities of APX were determined as follows; The reaction mixture for the peroxidase contained 50 mM potassium phosphate, pH 7.0, 0.5 ml fruit extract, 0.1 mM hydrogen peroxide, and 0.1 mM EDTA in a total volume of 1 ml (total reaction mixture). Then, the absorbance of the reaction was recorded 10 to 30 seconds at 290 nm after all the components were mixed. The decrease in absorbance at 290nm was recorded using an extinction coefficient of 2.8 mM−1·cm−1. The activity of ascorbate oxidized was then estimated.
Statistical analysis
The Statistical Package for Social Sciences Software (SPSS) version 16.0 was utilized for statistical analysis. All the analyses were in triplicates, ‘randomly selected from 40 fruits in each treatment’ and the standard error of the mean (SEM) was calculated. A one-way analysis of variance (ANOVA) was used to ascertain the treatment impact. Microsoft Excel 2016 was used to construct all of the graphs and charts. Duncan’s multiple range test (DMRT) at P<0.05 was used to distinguish significant differences between treatments.
Results and Discussion
Effect of salicylic acid treatments on the sugar content of African star apple fruits
During storage, the sugar content of C. albidum fruits changed considerably (P<0.05) in all analyzed samples. Total sugar content ranged from 97.13 to 609.76 mg/g, with reducing sugar content ranging from 25.29 to 66.24 mg/g and non-reducing sugar levels ranging from 66.4 to 543.52 mg/g (Table 1). In comparison to the other treated samples, the untreated negative control samples had the highest overall sugar content (609.76 mg/g), non-reducing sugar content (543.52 mg/g), and reducing sugar content (66.24 mg/g). The lowest total sugar contents were observed in distilled water treated samples with 97.13, 215.68, and 191.83 mg/g in 5, 10, and 15 min treatments, respectively.
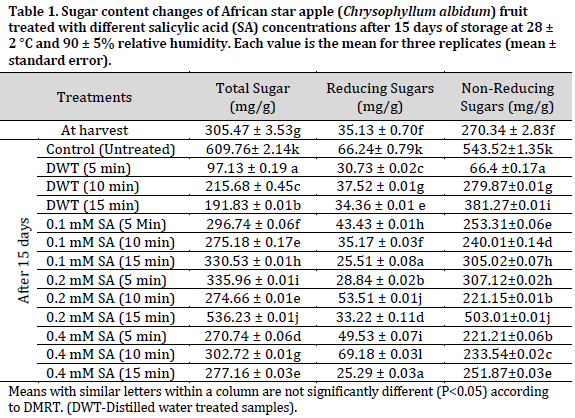
The metabolic degradation of complex carbohydrates into water-soluble sugars may be responsible for the rise in sugar content in some samples [24]. Ripening is thought to be responsible for the conversion of polysaccharides into water-soluble sugars, with sugars being further transformed into CO2 and alcohols as the ripening process progresses, resulting in the loss of sugar content in the fruits [24]. On the other hand, the delayed breakdown of complex carbohydrates into simple sugars is caused by slower respiration and overall metabolic rates. Salicylic acid extends storage life by suppressing ripening and senescence processes, preserving nutritional properties. The reduced sugar level in the treated fruits might be owing to the salicylic acid delaying effect on the ripening process. Salicylic acid was reported to extend the ripening phase and delay senescence in the apricot cultivar ‘Habi’ while stored at room temperature [24]. Furthermore, the findings of this research agree with the results presented by [9], who indicated that salicylic acid treatment caused a decrease in total sugar, reducing sugar, and non-reducing sugars compared to control samples. Salicylic acid was shown to be helpful in preserving total sugar content in pomegranate [25], strawberry [26], and banana [27] fruits owing to its ability to delay ripening, improve quality, and decrease postharvest losses of fruits and vegetables [28].
Impact of salicylic acid treatment on non-enzymatic antioxidant contents of African star apple fruits
There was an overall rise in phenolic content of all the fruits studied after 15 days of storage as compared to the fresh fruits in the current research. The phenolic content of salicylic acid-treated C. albidum fruits ranged from 12.81 to 15.45 mg GAE/g after 15 days of storage (Fig. 1 A). Fruits treated with 0.4 mM SA for 15 minutes had the highest phenolic content (15.45 mg GAE/g), whereas the fruits of the untreated negative control group had the lowest (2.34 mg GAE/g). The antioxidant activity of naturally occurring compounds in fruits and vegetables has long been known.
The phenolics are the most extensively distributed of these compounds and known for their scavenging properties of free radicals, hydroxyl radicals, superoxide with a single electron transfer [9]. Furthermore, fruits with phenolic compounds, such as C. albidum, contain antibacterial properties and are regarded to be bacteriostatic and fungistatic [16]. Additionally, polyphenols improve insulin sensitivity, decreasing the risk of type 2 diabetes [29]. Higher phenolics were also detected in SA-treated grapes [11], apricot [24], and broccoli sprouts [30], similar to the current findings. According to [9], the presence of phenolics in fruit cells may aid in the preservation of ascorbic acid levels.
The flavonoid concentrations in salicylic acid-treated C. albidium fruits ranged from 1.22 to 2.57 mg QE/g while the lowest value (0.97 mg QE/g) was recorded in the untreated control samples at the end of the storage period (Fig. 1 B). The fruits treated with 0.4 mM SA for 15 min had the highest total flavonoid content (2.57 mg QE/g). Flavonoids are phenolic-rich, water-soluble super antioxidants and free radical scavengers that protect cells from oxidative damage and exhibit significant anti-cancer and anti-inflammatory action [31]. The increase in flavonoids in SA-treated fruit compared to control is similar to the previously reported results in broccoli under SA treatment [30].
In this investigation, untreated control samples showed a reduction in ascorbic acid (vitamin C). On the other hand, positive control (DWT) and SA treatments showed an increased ascorbic acid level after storage period compared to its levels at harvest (Fig. 1 C). This observation might be due to cell wall breakdown during the ripening phase, which offers substrates for ascorbic acid synthesis as the ripening stage progresses [32]. A rise in ascorbic acid level indicates that the fruits are still ripening, while a drop indicates that the fruit is senescent [32]. [4] observed that the high ascorbic acid concentration of C. albidum fruit contributes significantly to the acidic taste of the fruit, particularly when it is not completely ripe and still mushy.
Antioxidants like ascorbic acid have been shown to help prevent nutritionally related disorders, including cancer, diabetes, coronary heart disease, and obesity [33]. According to the findings of this investigation, salicylic acid treatment enhanced the level of ascorbic acid over the storage period. The most significant amounts of ascorbic acid were found in C. albidum fruits treated with 0.4 mM SA for 15 minutes (59.38 mg/g). Therefore, SA treatments effectively delayed the degrading of ascorbic acid content. According to [9], this pattern of ascorbic acid retention might be related to a reduction in fruit respiration or oxidation of ascorbic acid content in the treated fruits with an airtight state, which decreased ascorbic acid loss.
Compared to the control group, salicylic acid-treated fruits retained higher contents of phenolic compounds and ascorbic acid. This observation agrees with previous reports that phenolic compounds might have a protective effect on ascorbic acid [9][34].
In this study, significant variations (P<0.05) were observed in carotenoid levels between the treated fruits, the control group, and the freshly picked fruity. At 15 days of storage, the fruits treated with 0.2 mM SA for 15 minutes had the highest mean value of carotenoids (0.69 mg/kg), while the positive controls (DWT) had the lowest results (Fig. 1 D).
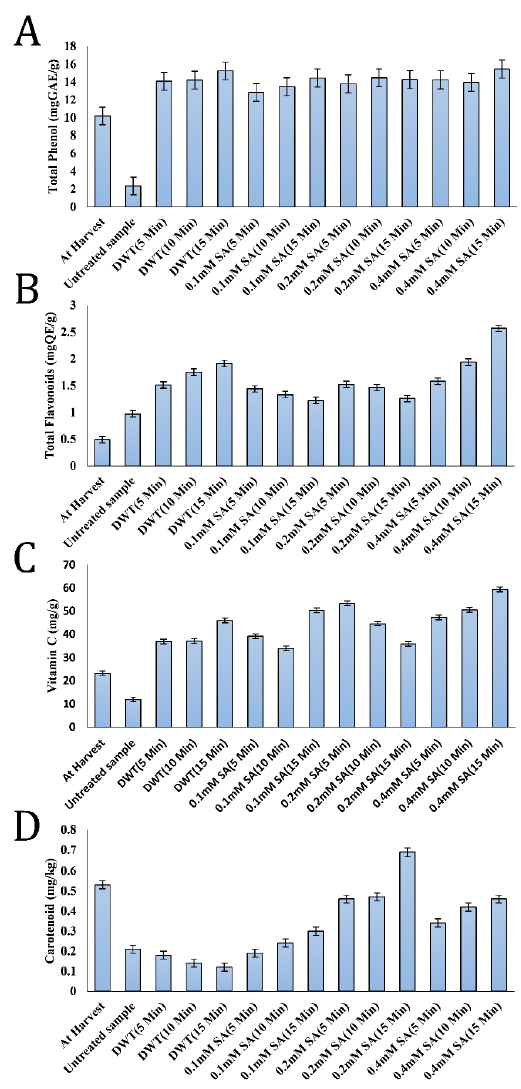
Except for the fruits treated with 0.2 mM SA for 15 minutes, which had an elevated value of carotenoids compared to fresh fruit, there was a general reduction in the value of carotenoid contents. In fact, storage conditions, such as temperature and light, influence carotenoids production and accumulation. Specific storage conditions may be employed as abiotic stress to boost carotenoids biosynthesis in postharvest plant tissues [35]. Furthermore, carotenoids in food matrices are sensitive to oxygen, peroxides, high temperature, light, and storage time [36]. Any of these elements may induce carotenoids destruction or isomerization. Therefore, controlling postharvest storage conditions is critical to improving carotenoids content and delaying their degradation in fruits and vegetables.
Impact of salicylic acid treatment on the enzymatic activities of African star apple fruits
Except for the untreated control sample, which exhibited increased activity, all samples showed an overall decline in catalase activity by the end of the storage period (Fig. 2 A). Salicylic acid-treated samples had catalase activity ranging from 1.19 to 2.88 IU/min. The distilled water tested has an IU/min range of 1.85 to 2.87. The untreated control sample had the maximum activity (7.78 IU/min), whereas the fruits treated with 0.4 mM SA for 5 minutes had the lowest (1.19 IU/min).
The highest SOD activity (90.20%) was observed in the 0.1 mM SA (15 min) treatment, whereas the lowest SOD activity (60.73%) was found in the untreated negative control (Fig. 2 B).
APX activities in this present study ranged from 0.02 to 0.06 µmol/min. There was no significant difference (P>0.05) in the value of APX activities among the freshly harvested fruits, untreated control sample, distilled water treated sample for 5 minutes, 0.1 mM SA (15 min), 0.2 mM SA samples (5 min), 0.4 mM salicylic SA for 10 and 15 minutes respectively (Fig. 2 C). The highest APX value was recorded in the sample treated with 0.2 mM SA for 10 min while the lowest was recorded in the fruits treated with distilled water for 10 and 15 minutes and 0.1 mM salicylic acid treated samples for 10 minutes.
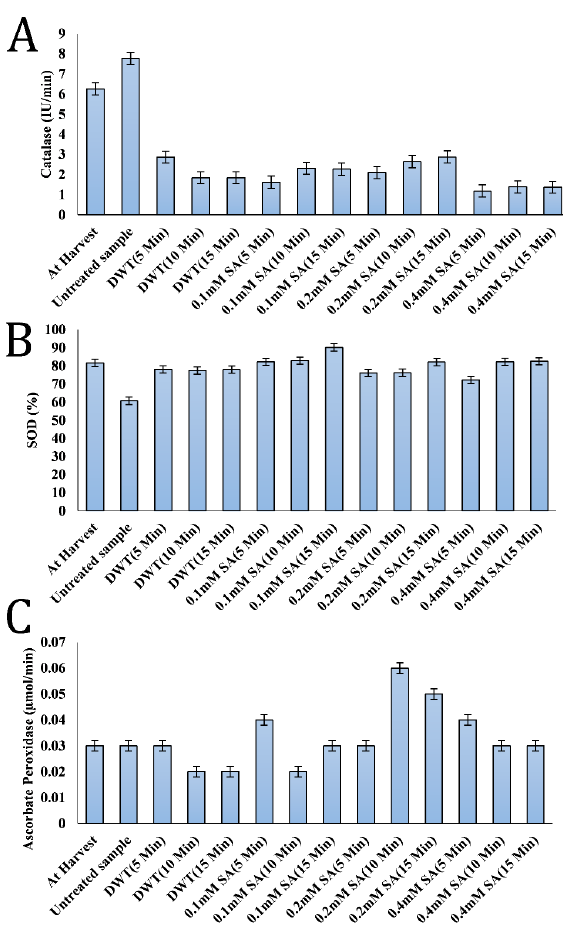
The decrease in salicylic acid-treated catalase activity was in contrast to the results of [37], who found that postharvest treatment of peach fruit with 1.0 mM SA for 10 minutes, alone or in conjunction with ultrasonic therapy, increased antioxidant enzyme activity (SOD, CAT, APX) [7]. However, the current results agree with [38], who reported the diminished catalase activity during the ripening phase of bananas under salicylic acid treatment.
Plants use the enzymes CAT, SOD, APX, polyphenol oxidase (PPO), phenylalanine ammonia-lyase (PAL), and peroxidase (POD) as means of protection against variety of stressors, including pathogen infestation. SOD may scavenge harmful free radicals by converting oxygen radicals (O2–) into H2O2 as a first line of defense [39].
It was reported that peach [40], cherry [41], and sugar apple (Annona squamosa L.) [42] fruits treated with salicylic acid demonstrated higher catalase (CAT), ascorbate peroxidase (APX), and superoxide dismutase (SOD) activities after storage. These observations are in accordance with the current results in terms of APX and SOD activities and contradict CAT results, which showed a decreased activity under SA treatments.
Conclusion
It can be deduced that salicylic acid treatment successfully preserved total phenol, flavonoid, ascorbic acids, and carotenoid contents while preserving ascorbate peroxidase and superoxide dismutase activities in African star apple C. albidum fruits under ambient storage conditions. However, total sugars, reducing sugars, non-reducing sugars, and catalase activities were lower in SA-treated fruits compared to that of the control. The C. albidum fruits immersed in 0.2 and 0.4 mM SA for 15 minutes had the best overall quality, with relatively good contents in all the tested parameters. Therefore, the use of salicylic acid as a postharvest treatment for C. albidum fruits might be a promising strategy to boost the fruit’s overall biochemical and nutritional quality under ambient storage conditions.
References
| 1 | Dandare SU, Mainasara BB, Magaji UF, Dandare A, Lailaba AA, Sadiq ME. In vitro antioxidant activity of Chrysophyllum albidum fruit. Nig. J. Basic Appl. Sci. 2017;25(1):17-22. DOI |
| 2 | Okwu C, Osazuwa ES, Igberaese SO. Nutritional and chemical composition of three fruit tastes of Chrysophyllum albidum (African Star Apple) in Nigeria. Int. J. Adv. Res. Sci. Eng. Technol. 2018;5:5033-7. |
| 3 | Lawal RT, Ishola AD, Sulaiman WK, Ajao FD, Adetuberu IA. Some aspects of physiochemical properties and evaluation of probiotics isolated from African star apple (Chrysophyllum albidum). Int. J. Multidiscip. Acad. Res. 2016;4:2309-18. |
| 4 | Bello AF, Henry AA. Storage effects and the postharvest quality of African star apple fruits (Chrysophyllum africanum) under ambient conditions. Afr. J. Food Sci. Technol. 2015;6:635-43. DOI |
| 5 | Oluwole OB, Odediran O, Ibidapo OP, Owolabi S, Chuyang Li, Garry S. Proximate composition, phytonutrients and antioxidant properties of oven dried and vacuum dried African star apple (Chrysophyllum albidum) products. Int J Nutr Food Sci. 2017;6:22-5. DOI |
| 6 | Panwar N, Rai PN, Kumar J, Mishra DS, Singh DP. Effect of different chemicals on litchi (Litchi chinensis Sonn.) cv. rose scented. J. Pharmacogn Phytochem. 2018;7(4):1418-22. |
| 7 | Aghdam MS, Asghari M, Babalar M, Sarcheshmeh MA. Impact of salicylic acid on postharvest physiology of fruits and vegetables. In Eco-friendly technology for postharvest produce quality. Academic Press. 2016:243-68. DOI |
| 8 | Moradinezhad F, Khayyat M. Effects of intermittent warming and prestorage treatments (hot water, salicylic acid, calcium chloride) on postharvest life of pomegranate fruit cv. ‘Shishe-Kab’ during long-term cold storage. Int. J. Hortic. Sci. Technol. 2014;1(1):43-51. DOI |
| 9 | Pila N, Gol NB, Rao TVR. Effect of postharvest treatments on physicochemical characteristics and shelf life of tomato (Lycopersicon esculentum Mill.) fruit during storage. Am. Eurasian J. Agric. Environ. Sci. 2010;9(5):470-9. |
| 10 | Ranjbaran E, Sarikhani H, Wakana A, Bakhshi D. Effect of salicylic acid on storage life and postharvest quality of grape (Vitis vinifera L. cv. Bidaneh Sefid). J. Fac. Agric. Kyushu Univ. 2011;56:263–9. |
| 11 | Khademi Z, Ershadi A. Postharvest application of salicylic acid improves storability of peach (Prunuspersicacv.Elberta) fruits. Int. J. Agric. Crop Sci. 2013;5:651-5. |
| 12 | Oguntimehin MO, Badejo AA, Enujiugha VN. Calcium chloride efficacy on physicochemical properties and microbial count of Chrysophyllum albidum- Linn fruit during storage. Turk J. Agric. Food Sci. Technol. 2022;10(2):235-43. DOI |
| 13 | Tilahun AT. Analysis of the effect of maturity stage on the postharvest biochemical quality characteristics of tomato (Lycopersicon esculentum MILL.) fruit. Int. Res. J. Pharm. Appl. Sci. 2013;3:180-6. |
| 14 | Singleton VL, Orthofer R, Lamuela-Raventos RM, Lester P. Analysis of total phenols and other oxidation substrates and antioxidants using Folin Ciocalteau reagent. Int. Methods in Enzymology (ed.). 1999:152-78. Academic Press. DOI |
| 15 | Akwu NA, Naidoo Y, Singh M. Cytogenotoxic and biological evaluation of the aqueous extracts of Grewia lasiocarpa: An Allium cepa assay. S. Afr. J. Bot. 2019;125:371-80. DOI |
| 16 | Asare IK, Okyere AA, Duah-Bissiw D, Ofosu DO, Darfour B. Nutritional and phytochemical constituents of the African star apple (Chrysopyllum albidum G. Don.). Ann. Food Sci. Technol. 2015;16:138-46. |
| 17 | Benderitter M, Maupoli V, Vergely C, Dalloz F, Briot F, Rochette L. Studies by electron paramagnetic resonance of the importance of iron in the hydroxyl scavenging properties of ascorbic acid in plasma: Effects of iron chelators. Fundam. Clin. Pharmacol. 1998;12:510-6. DOI |
| 18 | Alam MK, Rana ZH, Islam SN. Comparison of the proximate composition, total carotenoids, and total polyphenol content of nine orange-fleshed sweet potato varieties grown in Bangladesh. Foods. 2016;5:64. DOI |
| 19 | Osuntokun OT, Olumekun VO, Ajayi AO, Omotuyi IO, Olonisakin A. Assessment of in-vitro Antioxidant/Enzymes Inhibitory Potentials of Aframomum melegueta [Roscoe] K. Schum (Grains of Paradise) Leaf, Stem Bark, Seed Bark and Seed Extracts. Arch. Curr. Res. Int. 2020; 20(2):40-57. DOI |
| 20 | Geransayeh M, Sepahvand S, Abdossi V, Nezhad RA. Effect of thymol treatment on decay, postharvest life and quality of strawberry (Fragaria ananassa) Fruit CV ‘Gaviota. Int. J. Agron. Agric. Res. 2015;6(4):151-62. |
| 21 | Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem.1972; 247(10): 3170-5. |
| 22 | Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22(5):867-80. |
| 23 | Choudhary M, Manjhi J, Sinha A. Effect of drought stress in various enzymes of Pennisetum glaucum. Int. J. Appl. Sci. Biotechnol. 2015;3(1):134-8. DOI |
| 24 | Sartaj A, Tariq M, Kashif SA, Talat M, Amjad A. Effect of different concentrations of salicylic acid on keeping quality of Apricot cv. habiat ambient storage. J. Biol. Food Sci. Res. 2013;2(6):69-78. |
| 25 | Sayyari M, Babalar M, Kalantari S, Serrano M, Valero D. Effect of salicylic acid treatment on reducing chilling injury in stored pomegranates. Postharvest Biol. Technol. 2009;53(3):152-4. DOI |
| 26 | Babalar M, Asghari M, Talaei A, Khosroshahi A. Effect pre– and postharvest salicylic acid treatment on ethylene production fungal decay and overall quality of Selva strawberry fruit. Food Chem. 2007;105:449-53. DOI |
| 27 | Srivastava MK, Dwivedi UN. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 2000;158:87-96. DOI |
| 28 | Asghari M, Aghdam MS. Impact of salicylic acid on postharvest physiology of horticultural crops. Trends Food Sci Technol. 2010;21(10):502-9. DOI |
| 29 | Iheagwam PN, Onyeike EN, Amadi BA. Phytochemical and vitamin contents of Mangifera indica (mango) fruits subjected to ripening by artificial methods. Int. J. Environ. Agric. Biotech. 2019;4(3). DOI |
| 30 | Pérez-Balibrea S, Moreno DA, García-Viguera C. (2011). Genotypic effects on the phytochemical quality of seeds and sprouts from commercial broccoli cultivars. Food Chem. 2011;125(2):348-54. DOI |
| 31 | Okwu DE, Morah FN. Isolation and characterization of flavanone glycoside 4 I, 5, 7-trihydroxy flavanone rhamnoglucose from Garcinia kola Seed. J. Appl. Sci. 2007;7(2):306-9. DOI |
| 32 | Dandago MA, Gungula D, Nahunnaro H. Effects of postharvest dips on quality and storability of tomato fruits (Lycopersicon esculentum MILL) in Kura, Kano State, Nigeria. Pak. J. Food Sci. 2017;18:78-84. |
| 33 | Adepoju OT, Adeniji PO. Nutrient composition and micronutrient potential of three wildly grown varieties of African star apple (Chrysophyllum albidum) from Nigeria. Afr. J. Food Sci. 2012;6:344-51. DOI |
| 34 | Gharezi M, Joshi N, Sadeghian E. Effect of postharvest treatment on stored cherry tomatoes. J. Nutr. Food Sci. 2012;2(8). DOI |
| 35 | Ngamwonglumlert L, Devahastin S, Chiewchan N, Raghavan V. Plant carotenoids evolution during cultivation, postharvest storage, and food processing: A review. Compr. Rev. Food Sci. Food Saf. 2020;19(4):1561-604. DOI |
| 36 | Taleon V, Mugode L, Cabrera-Soto L, Palacios-Rojas N. Carotenoid retention in biofortified maize using different postharvest storage and packaging methods. J. Food Chem. 2017;232:60-6. DOI |
| 37 | Yang LY, Zhang JL, Bassett CL, Meng XH. Difference between chitosan and oligochitosan in the growth of Monilinia fructicola and control of brown rot in peach fruit. LWT-Food Sci. Technol. 2012;46(1):254-9. DOI |
| 38 | Barua S, Rahi T, Ullah E, Ghosh D, Ahmed S. Delay in fruit ripening: a promising approach for reduction of spoilage and use of hazardous chemicals in Bangladesh. Int. J. Agron. Agric. Res. 2015;6(4):163-73. |
| 39 | Li J, Mao L, Zhang Y, Zhang L, Jiang H. Phytochemical changes in mango fruit in response to Alternaria alternata infection. Czech J. Food Sci. 2018;36(3):227-32. DOI |
| 40 | Razavi F, Hajilou J, Aghdam MS. Salicylic acid treatment of peach trees maintains nutritional quality of fruits during cold storage. Adv. Hortic. Sci. 2018;32(1):33-40. DOI |
| 41 | Giménez MJ, Serrano M, Valverde JM, Martínez‐Romero D, Castillo S, Valero D, Guillen F. Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars. J. Sci. Food Agric. 2017;97(4):1220-8. DOI |
| 42 | Mo Y, Gong D, Liang G, Han R, Xie J, Li W. Enhanced preservation effects of sugar apple fruits by salicylic acid treatment during postharvest storage. J. Sci. Food Agric. 2008;88(15): 2693-9. DOI |
Cite this article:
Oguntimehin, M., Badejo, A., Enujiugha, V. The biochemical attributes of African star apple fruits are influenced by salicylic acid treatment during ambient storage. DYSONA – Applied Science, 2022;3(2): 56-67. doi: 10.30493/das.2022.339707
