Nagham A.R. Mushemesh 1*; Ansam R. Mahmoud 1; Duha K. Ressan 1; Ziyad T. Abdel-Baqi 2; Safaa M. Jassim 2
1, Industrial Microbiology Department, Food and Biotechnology Centre, Agricultural Research Directorate, Ministry of Science and Technology, Baghdad, Iraq
2, Chemistry Department, Agricultural Research Directorate, Ministry of Science and Technology, Baghdad, Iraq
E-mail:
Nagham.kenany70@gmail.com
Received: 20/08/2023
Acceptance: 28/09/2023
Available Online: 30/09/2023
Published: 01/10/2023

Manuscript link
http://dx.doi.org/10.30493/DLS.2023.412545
Abstract
The objective of this study was to assess the inhibitory activity of various plant extracts obtained through the aqueous extraction method against pathogenic bacteria. The experiment included cloves, nettle, chamomile, turmeric, fenugreek, rosemary, and garlic extracts at two concentrations (50 and 75 mg/ml). The agar well diffusion method was used to investigate the inhibitory potentials of the used extracts against three pathogenic bacterial isolates, namely Salmonella spp., E. coli, and Staphylococcus spp. The inhibition zone diameters of the aforementioned extracts were measured and subsequently compared with one another. The findings indicated that the garlic extract exhibited superiority when administered at a concentration of 50 and 75 mg/ml recording an average inhibition zone diameter of 14.18 and 14.28 mm, respectively. Moreover, garlic extract was notably more effective in inhibiting Staphylococcus spp. growth compared to other extracts at both tested concentrations. On the other hand, turmeric extract displayed a modest inhibitory performance against all three tested bacteria at both concentrations. The present findings exhibit promise in the development of novel and efficacious antibacterial agents targeting pathogenic bacteria. Nevertheless, further experimentation is required to ascertain the appropriate concentrations of plant extracts, refine the extraction technique, and integrate these extracts into in vivo trials.
Keywords: Plant extracts, Inhibition, Staphylococcus spp., Salmonella spp., E. coli
Introduction
According to the World Health Organization, bacterial infectious diseases rank prominently as one of the primary contributors to global mortality rates [1]. The aforementioned fact becomes increasingly alarming when taking into account the enduring issue of resistance to traditional antibiotics. Hence, there exists a critical need for the development of innovative and economically viable antibacterial interventions to effectively tackle the urgent healthcare demands on a global scale.
The emergence of antimicrobial-resistant bacterial species can be attributed to various factors, such as the widespread inappropriate utilization of antibiotics, inadequate effectiveness, limited solubility, as well as the extensive application of these antibiotics in animal production cycles. Hence, the issue of antibiotic resistance in both human and animal populations will persist until effective measures are implemented to eradicate it [2].
Plants possess a remarkable capacity to synthesize a diverse array of secondary metabolites, encompassing alkaloids, glycosides, terpenoids, saponins, flavonoids, quinones, and coumarins [3]. Thus, plant products have long served as a prolific reservoir for numerous medicinal compounds, offering viable alternatives to antibiotics. Therefore, the utilization of active substances derived from medicinal plants is still used for addressing diseases and delivering primary health care in developing nations [4]. Moreover, it is expected that there will be a wider and more extensive use of herbal-derived products in the future, provided that their effectiveness and safety are evaluated and approved through rigorous scientific examination [5].
Many researches examined the efficacy of plant extracts derived from different plant components such as flowers, fruits, twigs, roots, and stems. These extracts have shown potential as natural preservatives, capable of extending the shelf life of food products and maintaining their overall quality [6][7]. Additionally, these plant extracts have also been investigated for their potential in treating certain infectious diseases [8][9]
The objective of this study was to assess the inhibitory effects of aqueous extracts from seven herbal plants (cloves, nettle, chamomile, turmeric, fenugreek, rosemary, and garlic) at two different concentrations (50 mg/ml and 75 mg/ml), on the growth of three types of infectious bacteria: Staphylococcus spp., Salmonella spp., and Escherichia Coli.
Materials and Methods
Aqueous extracts preparation
The research was carried out at the Ministry of Science and Technology, the Agricultural Research Directorate, the Center of Bio and Food Technology (Baghdad, Iraq).
The aqueous plant extracts of cloves buds (Eugenia caryophyllus), nettle leaves (Urtica dioica), chamomile flowers (Matricaria chamomilla), turmeric rhizomes (Curcuma zedoaria), fenugreek seeds (Trigonella foenum-graecum), rosemary leaves and stems (Rosmarinus officinalis), and garlic cloves (Allium sativum) were prepared using a water extraction method. The dried botanical specimens were procured from a nearby market and finely ground before being stored in containers. The containers were then kept in a dry location at a temperature of 25 °C until the extraction process commenced. To prepare each extract, 20 grams of dried powder was taken and placed in a 500 ml beaker containing 150 ml of distilled water. The contents were then mixed using a magnetic shaker for 10 minutes and subsequently boiled on low heat for a duration of 2 hours. The mixture was subsequently allowed to stand for a duration of 24 hours at a temperature of 25 °C. Following this, the solution was subjected to filtration using two layers of tulle and filter paper, with the sediment being disregarded. The resulting filtrate was then centrifuged at 3000 rpm for a period of 15 minutes and the clear extract was transferred to evaporation containers. The containers were then subjected to a temperature of 45°C in an electric oven for several days to obtain the dry extracts. The fully dried extracts were then transferred into sterile containers and stored at 5 °C.
To prepare diluted extracts, 100 mg of dry extract was diluted in 1 ml of sterile distilled water. The resulting stock was then sterilized by filtration using MF-Millipore™ Membrane Filters (pore size 0.45 µm). Two dilutions (50 mg/ml and 75 mg/ml) were then prepared from each extract stock [10]. Subsequently, the diluted extracts were filtrated again as mentioned before.
Bacterial isolates preparation
The bacterial species used in the current study were Staphylococcus spp. (Gram-positive), Escherichia coli (Gram-negative), and Salmonella spp. (Gram-negative). These bacteria were isolated and identified previously [11]. McFarland standard (0.5) was used to standardize bacterial growth in order to achieve a bacterial cell concentration of 108 CFU/ml [12].
Inhibitory effect evaluation
The agar well diffusion method was employed to assess the antibacterial efficacy of the examined extracts [13]. Petri dishes containing Moller Hinton Agar (MHA) were inoculated with 100 μl of bacterial suspensions with a concentration of 108 CFU/ml. The inoculated plates were then allowed to set undisturbed for a duration of 10 minutes. The wells were created utilizing a sterile cork borer with a diameter of 6 mm. To each respective well, 100 μl of one of the extracts (either 50 mg/ml or 75 mg/ml) was added. The plates were left undisturbed for 30 minutes to allow for the diffusion of the extracts into the agar medium. Subsequently, the dishes were incubated at a temperature of 37°C for 18 hours. The antimicrobial activity was assessed by measuring the diameter of the inhibition zone in mm [14].
Statistical analysis
The experiment was conducted in triplicates using a completely randomized design (CRD). The data were subjected to analysis using GenStat 12 software, and the means were compared using Duncan’s test at 5% significance level.
Results and Discussion
The results indicated that the extracts at 50 mg/ml concentration exhibited an inhibitory effect on the bacteria under investigation, with an inhibition zone diameter ranging from 4.33 mm to 15.5 mm (Table 1). There were no significant variations observed in the average response of bacteria to the extracts. On the other hand, when comparing average extract effectiveness, the garlic extract exhibited the highest inhibition diameter (14.18 mm) followed by rosemary (12.52 mm), while turmeric and chamomile scored the lowest (6.23 mm and 6.78 mm, respectively).
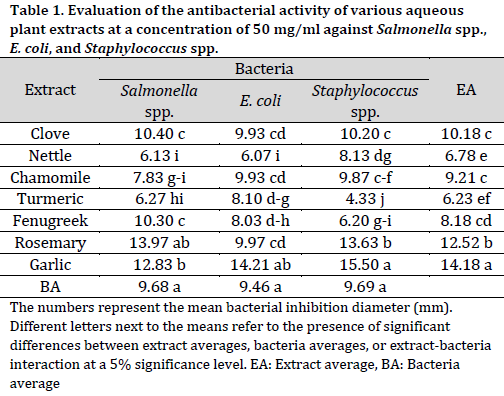
When considering individual inhibitory effects of the extracts at 50 mg/ml concentration, garlic extract exhibited greater efficacy in inhibiting the growth of Staphylococcus spp. and E. coli bacteria, while the rosemary extract showed superior inhibitory effects against Salmonella ssp. bacteria, with inhibition zone diameters of 15.5 mm, 14.21 mm, and 13.97 mm, respectively. On the other hand, turmeric and nettle extracts exhibited the least antibacterial activity against Staphylococcus spp. and Salmonella spp. bacteria. Additionally, the nettle extract displayed the lowest inhibition zone diameter of 6.07 mm against E. coli bacteria.
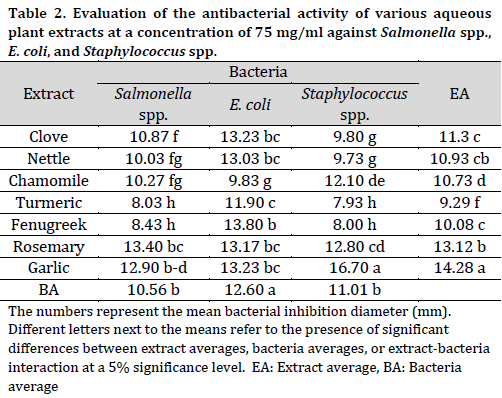
It was evident that the average inhibitory effect of all aqueous extracts at a concentration of 75 mg/ml was superior compared to those at a 50 mg/ml concentration. Additionally, it was observed that E. coli was more sensitive to the inhibitory effect of the aqueous extracts (average inhibition zone diameter of 12.6 mm) compared to the other two bacterial species (Table 2). Similar to its performance at a 50 mg/ml concentration, garlic extract at a 75 mg/ml concentration had a significantly higher inhibitory effect (average inhibition zone diameter of 14.28 mm) compared to other extracts. On the other hand, turmeric extract scored the lowest average inhibition zone diameter (9.29 mm). Among the 75 mg/ml extracts, the highest inhibition zone diameter was observed in the interaction between garlic extract and Staphylococcus spp. (16.7 mm).
Plants produce multiple bioactive compounds in order to protect themselves from pathogens in their natural habitats. These compounds (secondary metabolites) are deterrent or toxic to microorganisms [15]. Therefore, plant extracts have been used to treat common diseases and infections since the dawn of humanity. The bacterial inhibitory effects of garlic extract shown in the current work correspond with previous reports [16], which indicated that both Staphylococcus spp. and E. coli exhibited a high level of sensitivity to garlic extract. In fact, allicin, the main active compound is considered highly promising in treating untreatable infections whether used alone or in combination with other antibiotics [17]. Hence, garlic extracts have gained significance in the context of inhibiting drug-resistant Staphylococcus spp. bacteria.
Curcumin is known for its hydrophobic nature with poor water solubility [18]. This characteristic justifies the observed low inhibitory effect of turmeric aqueous extract against the tested bacterial species since its methanolic and n-hexane extracts have previously shown decent inhibition activity against Staphylococcus aureus and Salmonella typhi [19].
Various bacterial species exhibit varying degrees of inhibitory activity. The observed discrepancy could potentially be attributed to variations in laboratory extraction techniques, differences in the bacterial strains tested, variations in methods employed for isolating and preserving the bacterial strains, as well as factors such as the quality of the plant material and the manner in which it was collected and preserved. The increased inhibitory effect of all extracts at higher concentrations is expected since higher bioactive compounds are expected to present at higher concentrations. However, extract concentration should be extensively assessed in order to determine the optimum concentration while maintaining economic viability. This goal could be achieved by including a wide range of concentrations for the studied extracts in future research.
Conclusion
The presented results highlight the superiority of some aqueous plant extracts in inhibiting the growth of pathogenic Gram-negative and Gram-positive bacteria. Garlic extract was highly efficient in inhibiting Staphylococcus spp., while rosemary extract was superior in inhibiting Salmonella spp. In contrast, the inhibition efficacy of turmeric extract was found to be unsatisfactory, suggesting that the aqueous extraction method employed for this particular plant may not be suitable. Hence, additional tests are required to determine the optimum needed concentrations of plant extracts against specific bacterial strains, optimize the extraction method, and incorporate these extracts into in vivo experiments.
References
| 1 | WHO. Global Health Expenditure Database. 2023. [Cited 2023 Sep 23] Available from: Link |
| 2 | Elisha IL, Botha FS, McGaw LJ, Eloff JN. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement Altern. Med. 2017;17(1). DOI |
| 3 | Das K, Tiwari RK, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. Journal of medicinal plants research. 2010;4(2):104-11. |
| 4 | Verma S, Singh SP. Current and future status of herbal medicines. Vet. World. 2008;1(11):347. |
| 5 | Calapai G. European legislation on herbal medicines: a look into the future. Drug Saf. 2008;31:428-31. DOI |
| 6 | Negi PS. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012;156(1):7-17. DOI |
| 7 | Faraja D. Gonelimali, Jiheng Lin, Wenhua Miao, Jinghu Xuan, Fedrick Charles, Meiling Chen and Shaimaa R. Hatab. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018; 9(1). DOI |
| 8 | Navarro V, Villarreal ML, Rojas G, Lozoya X. Antimicrobial evaluation of some plants used in Mexican traditional medicine for the treatment of infectious diseases. J. Ethnopharmacol. 1996;53(3):143-7. DOI |
| 9 | El-Saadony MT, Zabermawi NM, Zabermawi NM, Burollus MA, Shafi ME, Alagawany M, Yehia N, Askar AM, Alsafy SA, Noreldin AE, Khafaga AF. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev. Int. 2023;39(4):2138-60. DOI |
| 10 | Jafeer KA, Kheirallah EA. Effect of Piper Nigrum fruit and Syzygium aromaticum roses extract on the some of Bacterial and fungal isolate growth. Al-Qadisiyah J. Pure Sci. 2016;21(2). |
| 11 | Hassan FM, Mahmood AR. Evaluate the efficiency of drinking water treatment plants in Baghdad City–Iraq. J. Appl. Environ. Microbiol. 2018;6(1). |
| 12 | CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. CLSI document M07-A9. Clinical and Laboratory Standards Institute. 2012;32. |
| 13 | Daoud A, Malika D, Bakari S, Hfaiedh N, Mnafgui K, Kadri A, Gharsallah N. Assessment of polyphenol composition, antioxidant and antimicrobial properties of various extracts of Date Palm Pollen (DPP) from two Tunisian cultivars. Arab. J. Chem. 2019;12(8):3075-86. DOI |
| 14 | CLSI. Performance standards for antimicrobial susceptibility testing: 25th informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute. 2015. |
| 15 | Kaur S, Samota MK, Choudhary M, Choudhary M, Pandey AK, Sharma A, Thakur J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants. 2022;28(2):485-504. DOI |
| 16 | Hawassa E, Sekota E. Anti-Bacterial effect of garlic (Allium sativum) against clinical isolates of Staphylococcus aureus and Escherichia coli from patients attending hawassa referral hospital, Ethiopia. J. Infect. Dis. 2016;2(2):18. |
| 17 | Cai Y, Wang R, Pei F, Liang BB. Antibacterial activity of allicin alone and in combination with β-lactams against Staphylococcus spp. and Pseudomonas aeruginosa. J. Antibiot. 2007;60(5):335-8. DOI |
| 18 | Kurien BT, Singh A, Matsumoto H, Scofield RH. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev. Technol. 2007;5(4):567-76. DOI |
| 19 | Gul P, Bakht J. Antimicrobial activity of turmeric extract and its potential use in food industry. J. Food Sci. Technol. 2015;52:2272-9. DOI |
Cite this article:
Mushemesh, N., Mahmoud, A., Ressan, D., Abdel-Baqi, Z., Jassim, S. The biological activity of some medicinal plant aqueous extracts in inhibiting the growth of pathogenic Gram-negative and Gram-positive bacteria. DYSONA – Life Science, 2023; 4(2): 66-70. doi: 10.30493/dls.2023.412545
