Samson O. Onemu 1; Rufus F. Ige 1; Mitefe O. Onemu-Metitiri 2; Paulinus O. Uyigue 1; Emmanuel I. Obeagu 3*
1, Department of Medical Laboratory Science, Achievers University, Owo, Nigeria
2, School of Sciences, Engineering and Environment, University of Salford, United Kingdom
3, Department of Medical Laboratory Science, Kampala International University, Uganda
E-mail:
emmanuelobeagu@yahoo.com
Received: 02/10/2023
Acceptance: 14/10/2023
Available Online: 15/10/2023
Published: 01/04/2024

Manuscript link
http://dx.doi.org/10.30493/DLS.2023.151009
Abstract
Asymptomatic bacteriuria is a commonly observed condition among pregnant women globally, with particularly high prevalence rates documented in African regions. Asymptomatic bacteriuria serves as an antecedent to the onset of urinary tract infection, which can lead to various difficulties during pregnancy. The objective of this study was to ascertain the prevalence of asymptomatic bacteriuria among pregnant women who sought care at the prenatal clinic in Akure, Nigeria. Samples of mid-stream urine were obtained from a cohort of pregnant women. The samples were inoculated onto standard bacteriological media and incubated at a temperature of 37°C for one night. The incubated samples were used to determine the prevalence of asymptomatic bacteriuria cases and to identify the specific bacterial etiology behind the occurrence of bacteriuria. Furthermore, an antibiotic susceptibility test was conducted to investigate the sensitivity of the isolated bacteria against common antibiotics. A notable prevalence of bacteriuria was seen in 360 out of 486 individuals, accounting for 74.1% of the studied population. The microbe with the highest prevalence was S. aureus, accounting for 23.6% of the total isolates followed by Klebsiella species and Escherichia coli. An increase in the asymptomatic bacteriuria rate was observed in younger ages, advanced gestational ages, and higher education levels. Fluoroquinolones were the most effective antibacterial agents with an average inhibition efficacy ranging between 78.1% and 89.9%. On the other hand, cephalosporins and other antibiotics showed moderate to low inhibition efficacy. The current observations indicate that the routine screening of pregnant women for asymptomatic bacteriuria should be implemented as a policy rather than being discretionary. Comprehensive sensitivity testing on individual bacteria is recommended to assist in the identification of safe and effective antibiotics for the management of asymptomatic bacteriuria in pregnant women.
Keywords: Asymptomatic bacteriuria, Urinary tract infection, Pregnancy, Staphylococcus aureus, Antibiotic susceptibility
Introduction
Asymptomatic bacteriuria (ASB) refers to the presence and proliferation of bacteria in the lower urinary tract without exhibiting the typical symptoms associated with urinary tract infection (UTI) [1]. ASB is a widely observed phenomenon on a global scale, impacting individuals of both genders. However, it is more frequently observed in women, particularly as they age [2]. This infection can also manifest in neonates and non-gravid females with preexisting urogenital anomalies [3] and is considered a significant predisposing factor for the development of cystitis, pyelonephritis, and pyonephrosis [1][4].
During pregnancy, ASB frequently occurs as a result of anatomical and physiological changes in the uterus, which undergoes significant enlargement and exerts substantial pressure on the urinary bladder and surrounding tissues [1][5][6]. These physiological changes occurring in the urogenital tract of pregnant women render them more susceptible to infections [6][7]. The presence of a urinary tract infection during pregnancy increases the risk of experiencing complications including preterm labor, neonatal sepsis, intrauterine growth restriction (IUGR), and intrauterine fetal demise or stillbirth [6][8]. Furthermore, ASB holds significant relevance in the context of pregnancy due to its potential progression to UTI in at least one-third of cases, leading to associated complications [9].
The occurrence of ASB during pregnancy exhibits significant variation across different population contexts. Several studies have reported rates ranging from 2% to 10% in developed economies [6][10], while certain regions in Asia have reported higher rates of 17% to 25.3% [11][12]. Studies conducted in Nigeria have reported a prevalence of ASB in pregnant women ranging from 15.1% to 86.6% [13-15].
In pregnant women, a significant portion of UTIs is attributed to the presence of a solitary bacterium. However, in cases of complicated UTIs, multiple microorganisms can be implicated, including those exhibiting greater drug resistance due to genetic mutations [5]. The determination of bacteriuria relies on quantitatively estimating the presence of bacteria in urine culture, following the Kass criteria established in 1956. These criteria aim to differentiate between true colonization of the urinary tract and accidental contamination of urine. According to the criteria, a count of 105 cfu/mL of urine indicates significant bacteriuria [16]. Alternatively, if the same organism is isolated in a repeat culture with a count of 102 cfu/mL and accompanied by the presence of at least 103 white blood cells (WBCs/mm3), it is also considered indicative of bacteriuria [17].
In the majority of healthcare facilities in developing nations, there is no standard practice of conducting regular and direct screening for ASB among antenatal patients [12][14]. However, the significance of conducting a thorough evaluation of urine culture has been recognized as a crucial factor in the identification of this condition as a UTI precursor, which is widely acknowledged as one of the most prevalent infections encountered in healthcare facilities worldwide [18]. The bacterial species commonly observed in ASB are classified within the enterobacterial group, with Escherichia coli being the most prevalent microorganism. Additional microorganisms that have been documented in the literature include Staphylococcus aureus, Pseudomonas aeruginosa, Proteus mirabilis, and Enterococcus faecalis [1][6][19]. The objective of this study was to ascertain the prevalence of asymptomatic bacteriuria (ASB) among pregnant women who visited the antenatal clinic in Akure metropolis, located in Ondo State, Nigeria. The assessment of bacterial diversity was conducted with consideration of the participants’ age, gestational age, and educational level. Additionally, an antimicrobial sensitivity test was conducted on the isolated bacteria in order to assess the prevalence of antibiotic-resistant strains among pregnant women exhibiting asymptomatic bacteriuria.
Material and Methods
Population and sample size
The study sample comprised of antenatal patients attending the specialist hospital in Akure, Ondo State. These patients provided informed consent and did not exhibit any symptoms of UTI. Additionally, they had not received any antimicrobial treatment in the two weeks leading up to the study. The study received ethical approval from the Ondo State Ministry of Health Research Ethics Committee, with reference number OSHREC.02/23/518, issued on March 02, 2023.
The sample size required to evaluate the prevalence of ASB was determined using a confidence level of 95% (Z=1.96). Given the available data on the average presence of ASB in pregnant women in Nigeria [13-15], a base prevalence rate of 50% was chosen. The sample size was determined using the formula:
N = Z2P(1-P)/d2
where Z=1.96, P=0.50 (50% prevalence), and d represents a fixed error limit of 0.05. Therefore, the minimum required sample size was 385. To account for the design effect, a 25% increase was applied to the initial sample size, resulting in a final required sample of 482 participants. In total, a cohort of 486 individuals was enlisted for the study.
Samples and data collection
Every enlisted participant was given instructions to collect a midstream specimen of urine (MSU). The samples that were gathered were appropriately labeled and were accompanied by relevant participant information (age, gestational age, and educational level). The specimens were stored in a refrigerated chamber and subsequently transferred to the laboratory for processing within 2 hours of collection.
Examination of Specimens
Each urine sample was thoroughly mixed and diluted at a ratio of 1:100 in sterile normal saline. Subsequently, 0.1 mL of the diluted sample was carefully transferred onto pre-dried plates of MacConkey agar (Oxoid CM 0007) or cysteine lactose electrolyte deficient, CLED agar (Oxoid CM 301). The plates were incubated at a temperature of 37°C for one night. The number of colonies (cfu) present on agar plates was counted and the average count of colonies was subsequently documented. Each sample exhibiting a count of 105 cfu/ml was deemed positive for ASB. The colonies were subsequently subjected to characterization and identification using the methodology outlined by [20].
A Chi-square test was used to determine the association between collected data categories (age, gestational age, and educational level) and ASB presence in participants.
Antibiotic susceptibility test
The in vitro susceptibility profile of the isolates to commonly used antibiotics was assessed using the disc diffusion method on Mueller-Hinton agar (Oxoid CM 033). The previously identified colonies were transferred using a sterile wire loop to a sterile normal saline medium and mixed to form a bacterial suspension. Subsequently, the suspension was evenly distributed across the surface of the Mueller-Hinton agar plate that had been prepared in advance. The antibiotic discs utilized in this study included Ampicillin (AMP) 10µg, Amoxicillin-clavulanate (AMC) 30µg, Ofloxacin (OFX) 10µg, Ciprofloxacin (CIP) 10µg, Levofloxacin (LVX) 10µg, Cefotaxime (CTX) 10µg, Cefuroxime (CXM) 25µg, Ceftriaxone (CRO) 30µg, Azithromycin (AZM) 15µg, and Gentamycin (GN) 10µg. The antibiotic discs were carefully positioned on the surface of the inoculated plates using sterile forceps. Subsequently, the plates were incubated for a period of 18-24 hours at a temperature of 37°C. The diameter of the inhibition zone was measured in mm. The bacterial isolates were considered susceptible to the antibacterial agent if they displayed an inhibition zone diameter greater than 12 mm [21]. The overall sensitivity of each bacterial species toward each antibiotic was determined by dividing the number of sensitive isolates by the total number of isolates of this species
Results
The diagnosis results showed that 74.1% (360 out of 486) of the participating pregnant women were diagnosed with ASB. Furthermore, all the studied categories (age group, gestational age, and educational level) were associated with ASB presence in test subjects. This association was confirmed by the statistically significant chi-squared values (p<0.05) obtained for all three variables. An increase in ASB cases was observed in younger ages, advanced gestational ages, and higher education levels (Table 1).
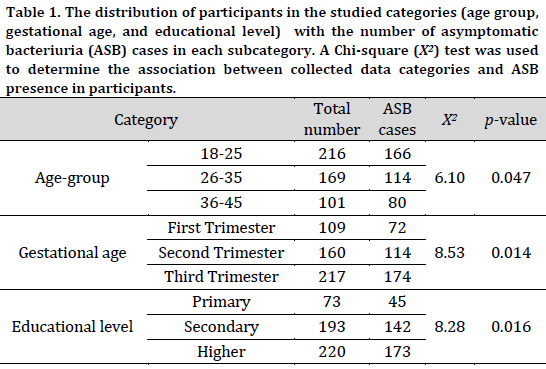
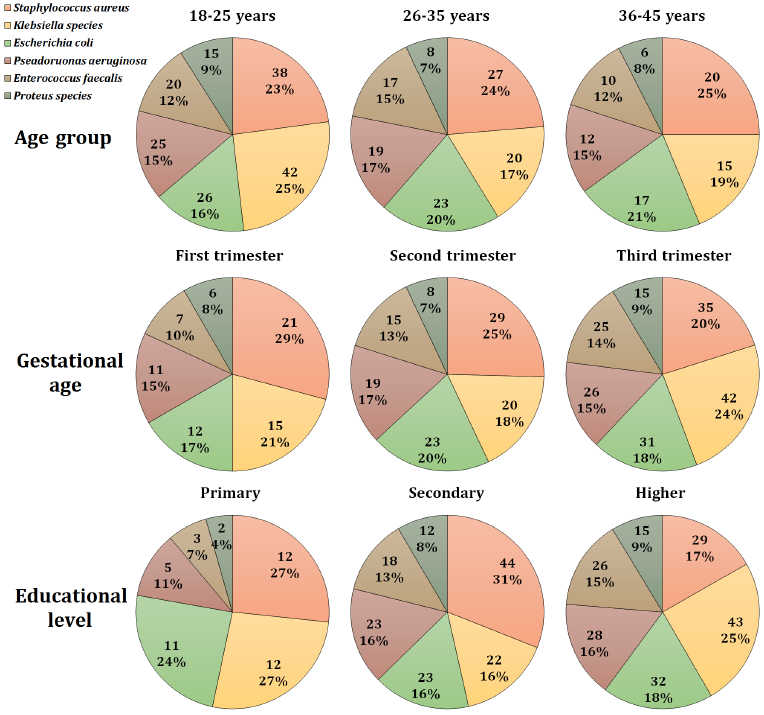
A total of six bacterial species were identified in the diagnosed samples, with Staphylococcus aureus being the most commonly isolated bacteria followed by Klebsiella species, accounting for 23.6% and 22.0% of the isolates, respectively. On the other hand, Proteus species were the least abundant isolated bacteria at 8.1% (Table 2). Based on this observation, Staphylococcus aureus was the most common bacterial isolate in most subcategories. However, Klebsiella species were the primary cause of ASB among 18-25 age group, third trimester group, and higher education group accounting for 25, 24, and 25% of total cases in each group, respectively (Fig. 1).
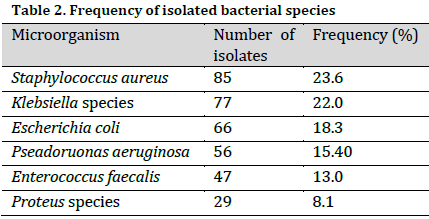
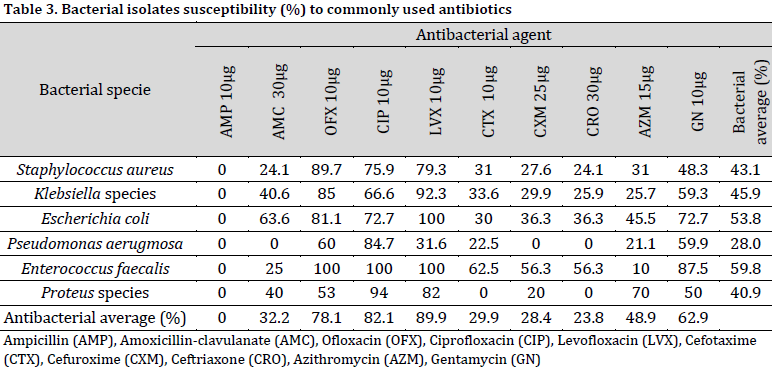
The susceptibility test revealed that most bacterial isolates (regardless of their specie) were susceptible to levofloxacin (89.9%), ciprofloxacin (82.1%), ofloxacin (78.1%), and azithromycin (62.9%). In fact, levofloxacin resulted in a total Escherichia coli and Enterococcus faecalis inhibition. Notably, none of the isolates demonstrated sensitivity to ampicillin at the used concentration. Moreover, the highest resistance (regardless of antibiotic type) was recorded in Pseudomonas aerugmosa isolates, as only 28% of the isolates (on average) demonstrated susceptibility to the used antibacterial agents (Table 3).
Discussion
The present study unveiled a substantial prevalence of ASB among pregnant women in the examined population (74.1%), which aligns with findings from previous research in Nigeria [13]. Staphylococcus aureus was the most frequently isolated bacteria (23.6%) followed by Klebsiella species and Escherichia coli. This finding deviates from previous studies, which have consistently reported E. coli as the predominant bacterium in ASB [1][6][19][22]. It is noteworthy to mention that a singular strain of bacteria was identified in each of the MSU samples, which aligns with the findings of [5], who similarly observed the presence of a single bacterial species in each case of ASB.
It is reported that advancing age is associated with an increased likelihood of acquiring asymptomatic bacteriuria [10]. However, a higher ASB rate was found in the youngest age group of pregnant women (18-25) compared to the other two age groups. This observation might be attributed to the inadequate habits of perineal hygiene among young pregnant women compared to older pregnant women [23].
The incidence of ASB during the initial trimester of pregnancy shows a decreased prevalence in comparison to the subsequent second and third trimesters. This discovery is consistent with previous results indicating that the anatomical and physiological changes experienced by pregnant women contribute to an increased susceptibility to urinary tract infections [6].
Existing literature has demonstrated that a higher level of education in pregnant women is associated with a protective impact against ASB [24]. However, other findings have also been reported, suggesting no significant association between educational level and the presence of asymptomatic bacteriuria [25]. Nevertheless, the present observations indicate that there is a greater prevalence of ASB among women with secondary and higher levels of education, as opposed to those with only a primary education. A previous study conducted by [26] also documented a comparable finding, indicating that women with preparatory and above educational levels exhibited a threefold increase in the incidence of UTI compared to women with no formal education. The present observations present a perplexing situation, as it is anticipated that highly educated women will exhibit a notably higher degree of adherence to hygienic practices in comparison to their less educated counterparts. The underlying factors contributing to these findings may be multifaceted and necessitate a comprehensive examination of the behavioral patterns and socioeconomic circumstances of women within the analyzed sample. Alternatively, it is plausible to provide more straightforward rationales, such as the heightened utilization of public and communal amenities by highly educated women within their educational and occupational settings. The persuasiveness of this theory is enhanced by the fact that a majority of the individual bacterial species behind ASB in the current work may be identified and transferred via communal objects [27][28].
The results of susceptibility tests indicated that fluoroquinolones (Ofloxacin, Ciprofloxacin, and Levofloxacin) exhibited the highest efficacy (ranging from 78.1% to 89.9%) in preventing the growth of the isolated bacteria in an in vitro setting. Despite the positive prospects, it is not advisable to administer fluoroquinolones to pregnant women, particularly in the initial trimester of pregnancy, due to their correlation with reduced rates of live births and increased rates of spontaneous abortion [29]. The cephalosporins and other antimicrobial drugs exhibited reduced rates of inhibition ranging from 23.8% to 62.9%, whereas ampicillin showed no inhibitory efficacy at all. The aforementioned findings are cause for concern and necessitate the formulation of novel treatment approaches within the examined demographic in order to mitigate the risk of subsequent UTI occurrence and potential exacerbation of health issues.
Conclusion
The present study demonstrated a significant prevalence of asymptomatic bacteriuria among pregnant women in the group under investigation. Bacteriuria occurrence was found to be associated with age group, gestational age, and educational level of women. The rates of asymptomatic bacteriuria in the study population are indicative of the need for regular screening of pregnant women as a measure to decrease or eliminate urinary tract infections caused by asymptomatic bacteriuria, and to mitigate the potential pregnancy problems that may ensue. It is advisable to perform comprehensive sensitivity testing on each bacterium in order to facilitate the identification of the antimicrobial agent that is both highly effective and minimally harmful in pregnant women with asymptomatic bacteriuria.
References
| 1 | Azami M, Jaafari Z, Masoumi M, Shohani M, Badfar G, Mahmudi L, Abbasalizadeh S. The etiology and prevalence of urinary tract infection and asymptomatic bacteriuria in pregnant women in Iran: a systematic review and Meta-analysis. BMC Urol. 2019;19:1-5. DOI |
| 2 | Luu T, Albarillo FS. Asymptomatic bacteriuria: prevalence, diagnosis, management, and current antimicrobial stewardship implementations. Am. J. Med. 2022;135(8): e236-44 DOI |
| 3 | Nicolle LE, Gupta K, Bradley SF, Colgan R, DeMuri GP, Drekonja D, Eckert LO, Geerlings SE, Köves B, Hooton TM, Juthani-Mehta M, Knight SL, Saint S, Schaeffer AJ, Trautner B, Wult B, Siemieniuk R. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2019;68(10):e83-110. DOI |
| 4 | Shaffer K, Bach J, Chun R. Prospective study evaluating the incidence of bacteraemia and bacteruria in afebrile and febrile neutropaenic dogs undergoing chemotherapy. Vet. Med. Sci. 2016;2(4):281-94. DOI |
| 5 | Nóbrega MM, Auge AP, de Toledo LG, da Silva Carramão S, Frade AB, Salles MJ. Bacteriuria and urinary tract infection after female urodynamic studies: risk factors and microbiological analysis. Am. J. Infect. Control. 2015;43(10):1035-9. DOI |
| 6 | Sheppard M, Ibiebele I, Nippita T, Morris J. Asymptomatic bacteriuria in pregnancy. Aust. N. Z. J. Obstet. Gynaecol. 2023. DOI |
| 7 | Bose AM, Sreekumary PK, Pulikkottil SK. Microbiological profile of asymptomatic bacteriuria in pregnancy. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017;6(4):1352-62. DOI |
| 8 | Cotton E, Geraghty R, Umranikar S, Saeed K, Somani B. Prevalence of asymptomatic bacteriuria among pregnant women and changes in antibiotic resistance: a 6-year retrospective study. J. Clin. Urol. 2022. DOI |
| 9 | Smaill FM, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst. Rev. 2019(11). DOI |
| 10 | Givler DN, Givler A. Asymptomatic bacteriuria. StatPearls [Internet]. 2023. [Cited 2023 Oct 8] Available from: Link |
| 11 | Prasanna B, Naimisha M, Swathi K, Shaik MV. Prevalence of asymptomatic bacteriuria in pregnant women, isolates and their culture sensitivity pattern. Int. J. Curr. Microbiol. App. Sci. 2015;4(8):28-35. |
| 12 | Singh L, Dubey R, Singh S, Goel R, Nair S, Singh PK. Measuring quality of antenatal care: a secondary analysis of national survey data from India. BJOG: Int. J. Obstet. Gynaecol. 2019;126:7-13. DOI |
| 13 | Akerele, P. Abhulimen, F. Okonofua J. Prevalence of asymptomatic bacteriuria among pregnant women in Benin City, Nigeria. J. Obstet. Gynaecol. 2001;21(2):141-4. DOI |
| 14 | Imade PE, Izekor PE, Eghafona NO, Enabulele OI, Ophori E. Asymptomatic bacteriuria among pregnant women. N. Am. J. Med. Sci. 2010;2(6):263. DOI |
| 15 | Ezeome IV, Ikeme AC, Okezie OA, Onyebueke EA. Asymptomatic bacteriuria (ASB) in pregnant women in Enugu, Nigeria. Trop. J. Obstet. Gynecol. 2006;23:12-3. DOI |
| 16 | Glaser AP, Schaeffer AJ. Urinary tract infection and bacteriuria in pregnancy. Urol. Clin. 2015;42(4):547-60. DOI |
| 17 | Najar MS, Saldanha CL, Banday KA. Approach to urinary tract infections. Indian J. Nephrol. 2009;19(4):129-39. DOI |
| 18 | Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019;11. DOI |
| 19 | Greve VH, Greve T, Helmig RB. Bacteriuria in pregnancy in a Danish contemporary cohort of women. Infect. Dis. Obstet. Gynecol. 2020. DOI |
| 20 | Cowan ST. Cowan and Steel’s manual for the identification of medical bacteria. Cambridge university press. 1993. |
| 21 | Wayne A. Clinical and Laboratory Standards Institute; CLSI. 2011. Performance standards for antimicrobial susceptibility testing. 20th Informational Supplement. CLSI document. 2017. |
| 22 | Sonkar N, Banerjee M, Gupta S, Ahmad A. Asymptomatic bacteriuria among pregnant women attending tertiary care hospital in Lucknow, India. Dubai Med. J. 2021;4(1):18-25. DOI |
| 23 | Edae, M., Teklemariam, Z., Weldegebreal, F. and Abate, D., 2020. Asymptomatic bacteriuria among pregnant women attending antenatal care at Hiwot Fana specialized university hospital, Harar, eastern Ethiopia: magnitude, associated factors, and antimicrobial susceptibility pattern. Int. J. Microbiol. 2020. DOI |
| 24 | Tchente Nguefack C, Okalla Ebongue C, Nouwe Chokotheu C, Ebong Ewougo C, Nana Njamen T, Mboudou E. Clinical presentation, risk factors and pathogens involved in bacteriuria of pregnant women attending antenatal clinic of 3 hospitals in a developing country: a cross sectional analytic study. BMC Pregnancy Childbirth. 2019;19:143-8 DOI |
| 25 | Labi AK, Yawson AE, Ganyaglo GY, Newman MJ. Prevalence and associated risk factors of asymptomatic bacteriuria in ante-natal clients in a large teaching hospital in Ghana. Ghana Med. J. 2015;49(3):154-8. DOI |
| 26 | Younis N, Khattab H, Zurayk H, El-Mouelhy M, Amin MF, Farag AM. A community study of gynecological and related morbidities in rural Egypt. Stud. Fam. Plann. 1993:175-86. DOI |
| 27 | Siranosian BA, Brooks EF, Andermann T, Rezvani AR, Banaei N, Tang H, Bhatt AS. Rare transmission of commensal and pathogenic bacteria in the gut microbiome of hospitalized adults. Nat. Commun. 2022;13(1):586. DOI |
| 28 | Giannini MA, Nance D, McCullers JA. Are toilet seats a vector for transmission of methicillin-resistant Staphylococcus aureus?. Am. J. Infect. Control. 2009;37(6):505-6. DOI |
| 29 | Acar S, Keskin-Arslan E, Erol-Coskun H, Kaya-Temiz T, Kaplan YC. Pregnancy outcomes following quinolone and fluoroquinolone exposure during pregnancy: a systematic review and meta-analysis. Reprod. Toxicol. 2019;85:65-74. DOI |
Cite this article:
Onemu, S., Ige, R., Onemu-Metitiri, M., Obeagu, E. The prevalence of asymptomatic bacteriuria in pregnant women in Akure, Ondo State, Nigeria. DYSONA – Life Science, 2024;5(1): 1-8. doi: 10.30493/dls.2023.151009
