Catherine Y. Babatuyi 1*; Abosaede M. Adisa 2; Temitope C. I. 3; Victor N. Enujiugha 1
1, Department of Food Science and Technology, Federal University of Technology, P.M.B.704, Akure, Nigeria
2, Department of Food Science and Technology, Joseph Ayo Babalola University Ikeji-Arakeji, Nigeria
3, Department of Food Science and Technology, Federal Polytechnic, Offa, Nigeria
E-mail:
cybabatuyi@futa.edu.ng
Received: 15/11/2022
Acceptance: 18/04/2023
Available Online: 19/04/2023
Published: 01/07/2023

Manuscript link
http://dx.doi.org/10.30493/DAS.2023.369327
Abstract
The quest to develop the production of sourdough flour from fonio whole grain is gaining increased attention recently due to its enhanced nutritional and health benefits compared with whole wheat flour. In this research, white and black fonio seeds were oven-dried at 50 °C for 8 h, milled separately into fine flour, sieved, reconstituted with water, and divided into three portions each. Two portions were separately fermented with Lactobacillus fermentum [with white fonio (WFF) and black fonio (BFF)] and Lactobacillus delbrueckii [with white fonio (WFD) and black fonio (BFD)]. The last portions of each type were set as controls [white fonio (WFC) and black fonio (BFC)] and fermented without starter cultures. All samples were fermented at 25 °C for 48 h, freeze-dried, milled, and stored inside airtight containers for further analysis. The results indicated that black fonio samples had higher total microbial loads (bacterial and yeast count), total crude protein, carbohydrate, and reduced anti-nutrient. In contrast, the white fonio favored the growth of lactic acid bacteria (LAB), with higher crude fiber, crude fat, mineral elements, and antioxidant contents. fermenting with Lactobacillus fermentum resulted in the highest free radicals scavenging activity followed by Lactobacillus delbrueckii. The pasting assessment and mineral content analysis showed that black fonio was superior. It can be deduced that both fonio types had their advantages and the use of LAB-mediated fermenting can enhance the characteristics and health benefits of fonio sourdough.
Keywords: Fonio, Sourdough, Fermentation, Lactobacillus, Millet
Introduction
Spontaneous “sour” dough fermentation is one of the earliest cereal fermentation methods known to man. In this technique, the dough is leavened to generate a more gaseous dough and more aerated bread. Due to its distinctively high-quality properties, the use of this dough is essential for a wide range of traditional sourdough goods, particularly for baking [1]. Lactic acid bacteria (LAB) species are often dominant in sourdough fermentation, which is a complex biological process [2].
Nowadays, consumers are highly concerned about the health aspects of the food they eat. Therefore, functional food items, such as sourdough-derived foods, are becoming increasingly popular due to their health benefits. Successful food products, on the other hand, must meet customers’ expectations for safety and optimal sensory characteristics [3]. Thus, Sourdough bread production technology is rapidly developing in recent years to obtain enhanced flavor, texture, and shelf life.
Cereals are a significant source of protein, dietary fiber (DF), vitamins, and bioactive substances. The outer layers of the grains contain the highest concentrations of the main health-protective substances, including oligosaccharides, phytochemicals, and DF [4]. According to several studies, the Western diet contains less than the daily recommended intake (25 to 30 g) of DF, and this has led to an excessive number of diseases, ranging from dental caries to obesity [5]. Therefore, wheat, barley, and oat brans have been incorporated in doughs to increase DF intake [6] and for the added nutritional value [7-9].
Fonio (Finger millet) is produced in Asia, Africa, and India and it is also known as fundi, findi, hungry rice, and Asian millet [10]. This cereal crop is resistant to viruses and pests with similar nutritional makeup compared to other cereal crops. Fonio is prepared and eaten in different indigenous forms such as tuo (tuwo i.e. in form of boiled rice with stew), djouka, couscous, gwete, acha-jollof, and kunuacha. Although it is considered native to Western Africa, Fonio attracted worldwide interest during the European Acha Project, which was run by the French Agricultural Research Center for International Development [11]. Since then, studies have demonstrated that the germination and fermentation of millets significantly lower the levels of anti-nutrients (such as phytate and tannin), promote efficient protein and starch hydrolysis, and increase mineral bioavailability in food products [12]. Furthermore, studies have reported that finger millets contain 5-8% protein, 1-2% ether extractives, 65-75% carbohydrates, 15-20% dietary fiber, 2.5-3.5% minerals, and are rich in polyphenols and calcium with high methionine content [13]. Therefore, fonio food products can assist in the management of diabetes, cardiovascular, celiac, cancer, aging, microbial and inflammatory diseases [1][11].
Lactic acid bacteria (LAB) have been used in food production for more than 6000 years (since, at least, 4000 BC), especially in dairy products [14]. These microorganisms dominated fermented plant products such as cereals, grains, and millet [15][16]. The lactobacilli are gastrointestinal tract (GIT) microorganisms necessary for maintaining and possessing probiotic activities in human digestion [17]. LAB-fermented foods are easily digestible and broken down in addition to their high antimicrobial substances content which assists in the prevention and inhibition of pathogenic bacterial growth [15]. Additionally, LAB presence in food is reported to reduce serum glucose, increase antioxidant activity, and alleviate cardiovascular and diabetes disorders [16][18].
Taking into consideration the previously mentioned points, choosing the right LAB species might be an influential factor in sourdough production. However, there is no available information regarding the influence of bacterial species type (Lactobacillus fermentum and L. delbrueckii) in the fermentation of fonio. Therefore, the goal of the current study is to determine how different starter bacteria can influence the chemical and pasting characteristics of fonio.
Materials and Methods
Collection of materials
White fonio (Acha) (Digitaria exili) and black fonio (Iburu) (Digitaria iburua) harvested within 6–8 weeks were obtained from a farm in Kaduna State, Nigeria. The fermenting microorganisms were obtained from Bato Laboratory in Lagos State, Nigeria. The fonio seeds were verified by the Crop, Soil, and Pest Management Department of the Federal University of Technology, Akure, Nigeria.
Reagents and chemicals
Chemicals of analytical grade were purchased from Sigma-Aldrich (Germany and USA). All the solutions were prepared with Deionized water. All the microbiological media such as Potato Dextrose Agar (PDA), deMan, Rogosa, Sharpe Agar (MRS), and Nutrient agar were obtained from Biotech (USA).
Microbial identification and characterization
The lactic acid bacteria were isolated using standard microbiological techniques. The isolates’ identities were verified using Bergery’s manual of identification [19] by examining the morphological and cultural characteristics of the colony formation. The identified Lactobacillus plantarum and Lactobacillus fermentum were isolated from deMan Rogosa Sharpe (MRS) broth and kept at 10 °C. Each Lactobacilli sp. broth culture was washed in 0.1 M potassium phosphate buffer three times before being centrifuged at 6000 rpm for 10 min using a refrigerated centrifuge (ThrmoFisher Scientific Sorvall ST16, USA). The cells were then re-suspended in another 20 mL of sterile 0.1 M potassium phosphate (pH 7.0), and standardized using a spectrophotometer (Model ST-VS-721, Hongkong) at a wavelength of 600 nm according to [20].
Processing of fonio flours
Both white and black seeds were sorted and cleaned to remove extraneous particles. The seeds were washed, drained, and dried in an oven for 8 h at 50°C. Subsequently, the dried seeds were grounded into flour and sieved separately to obtain fine and smooth flouraccording to [21]. The prepared flours were kept in hermetically sealed containers for future use.
Sourdough production
Sourdough was developed using the paste of fonio flours (white or black) as a substrate in a ratio of 1:1 (flour: water) and producedaccording to the method described by [22]. Initial Fermentation of the substrate with L. fermentum and L. delbrueckii as single and combined (1:1) starter was carried with the addition of water for 48 h at 25°C. After that, the sourdough was left to ferment for another 8 h at 25 °C before being freeze-dried and stored.
Microbial determination
For microbial tests, diluted saline suspensions of the sourdoughs were prepared. For that purpose, 0.85 g of sodium chloride was dissolved in 100 ml of distilled water. Then, 9 ml of the solution was added into MacConkey bottles, which were autoclaved at 121°C for 15 min. The bottles were cooled after sterilization, and one gram of sourdough was added to make 10-1 dilution. 1 ml from dilution 10-1 was taken into another bottle to make 10-2 dilution and again to make 10-3 dilution. Petri dishes were then prepared by dispensing 1 ml of the diluted sourdough suspensions into a sterilized petri dish.
Total bacterial count
Nutrient agar (NA) of 2.8 g was added to 100 ml of distilled water and autoclaved at 121°C for 15 min. Already cooled (45°C) molten agar was poured on prepared Petri dishes, rocked for even distribution, allowed to solidify, inverted, and incubated at 37 °C for 24 h before the colony count was inspected using a colony counter.
Total yeast count
Potato Dextrose Agar (PDA) (3.9 g) was added to 100 ml of distilled water and autoclaved at 121°C for 15 min. Already cooled (45°C) molten agar was poured on prepared Petri dishes, rocked for even distribution, allowed to solidify, inverted, and incubated at 25 °C for 72 to 120 h before the colony count was inspected using a colony counter.
Lactic acid bacterial count
deMan Rogosa Sharpe (MRS) (6.3 g) was added to 100 ml of distilled water and autoclaved at 121°C for 15 min. Already cooled (45°C) molten agar was poured on prepared Petri dishes, rocked for even distribution, allowed to solidify, inverted, and incubated at 37 °C for 24 to 48 h in a gas jar with a lighted candle to create anaerobic condition before the colony count was inspected using colony counter.
Chemical analyses
Proximate composition determination
Proximate content (%) for crude fat, protein, fiber, moisture, and ash of the microbial fermented sourdough was determined as described in [23]. Carbohydrate (CHO) content was determined as the remaining of other proximate compositions:
Carbohydrate (%) = 100- (Fat + Protein + Fiber + Moisture)
The energy value was calculated as:
Energy value (kJ/100g) = (Crude fat × 9) + (CHO × 4) + (Crude protein × 4)
Anti-nutrient composition determination
Determination of oxalate, tannin, phytate, and saponin of microbial fermented sourdough samples was done according to [12][23]
Mineral composition determination
The mineral composition of microbial fermented sourdough samples was determined using Atomic Absorption Spectrophotometer (AAS Model SP9) for calcium (Ca), iron (Fe), and zinc (Zn), and using flame photometer (FF902 UK) for potassium (K) and sodium (Na), while AOAC standard method was used to quantify NaCl as described by [12].
Antioxidants assay
Free radical scavenging ability
DPPH (2,2-diphenyl-1-picrylhydrazyl) method was used to estimate the free radical scavenging capacity of the produced sourdoughs [24]. First, 0.4 mM DPPH methanolic solution of 1 mL was added to 1 mL of flour extract (1:9 dough:water w/v). The assays were then incubated in the dark at room temperature for 30 min. The absorbance of the left (unscavenged) DPPH was determined at 516 nm. DPPH scavenging ability of flour samples was then estimated compared to prepared standards, while ethylene diamine tetraacetic acid (EDTA) serves as a control standard.
Total phenolic content determination
The total phenolic content of each microbial fermented sourdough was determined according to the method of [25]. Each flour extract (1:9 dough: water w/v) was diluted with 2.5 mL of 10% Folin–Ciocalteau reagent (vol/vol) before the addition of 2.0 mL 7.5% sodium carbonate and incubation at 45 °C for 40 min. The absorbance of each sample was measured with a spectrophotometer at 765 nm compared to gallic acid standards. Then, the total phenolic content was estimated and stated in gallic acid equivalent (GAE).
Determination of total flavonoid content
The method described by [26] was followed to determine the total flavonoid content in the prepared sourdoughs. For that purpose, 0.5 g of each of the microbial fermented sourdough was diluted with a mixture of methanol (0.5 mL), 10% AlCL3 (50 µL), 1 M potassium acetate (50 µL), and distilled water (1.4 mL). These mixtures were incubated for 30 min at 25°C and the absorbance of each sample was measured at 415 nm and compared with quercetin standards. The total flavonoid content was then estimated and stated in quercetin equivalent (QE).
Pasting characteristics
Microbial fermented sourdoughs were assessed using Rapid Visco Analyzer (RVA) according to [27]. Five grams of each sourdough was added to distilled water (25 mL) in a canister. The substrates were then carried into the canister’s water surface after inserting the canister analyzer piston. The piston surface was forcefully moved shakily in undulation movement severely to achieve a smooth flour sample. The canister and its paddle were rigidly thrust to the paddle coupling for proper fitting. The sourdough samples became sticky as a result of the high thick-flowing consistency and were subjected to controlled shear and high temperature to be stabilized and cooled to show any setback throughout gelatinization. The watery mixture was reheated to 95 °C from 50 °C before cooling back to 50 °C for 3 min. The pasting measurements were determined using Thermocline software (Warriewood, Australia).
Statistical analysis
The experiments were carried out in triplicates using a completely randomized design and the data obtained were subjected to analysis of variance (ANOVA) and means were separated using New Duncan’s Multiple Range Tests (NDMRT) using Statistical Package for Social Science (SPSS) version 21.0 (IBM Inc., USA) with data presented as Mean ± Standard deviations.
Results and Discussion
Microbial population of fonio sourdoughs
The total bacterial count ranged from 1.73×105 to 8.86×105 cfu/g, with the black fonio sample fermented with Lactobacillus fermentum (BFF) having the highest (8.86×105cfu/g) and white fonio fermented with Lactobacillus delbrueckii (WFD) having the least counts (1.73×105 cfu/g) (Table 1). It was also observed that sourdough fermented with L. fermentum had higher bacterial count compared to those fermented with L. delbrueckii. The total yeast count obtained ranged from 1.74×105 to 7.10×105 cfu/g. Black fonio sample fermented with L. delbrueckii (BFD) had the highest yeast count (7.10×105 cfu/g), while BFF had the least (1.74×105 cfu/g). LAB count ranged from 3.36×105 in BFF to 5.53×105 cfu/g in WFC. The difference in LAB could be attributed to the different Lactobacillus strains used for fermentation, as it appears that L. delbrueckii and control fermentation (no starter) made the medium suitable for LAB growth. On the other hand, L. fermentum starter resulted in a higher yeast count compared to L. delbrueckii. In general, the microbial loads of the samples were high, similar to other reports [27]. This could be a result of inherent microorganisms present in the fonio seeds as well as the environmental conditions during and after fermentation.
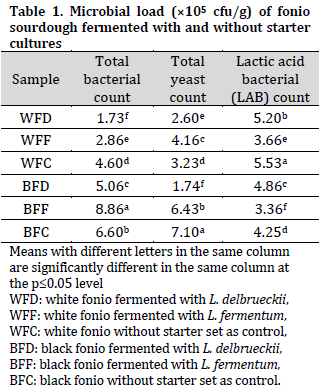
Proximate compositions of fonio sourdoughs
There was a significant difference in the moisture content of the samples which ranged from 12.78% in BFC to 15.62% in WFF. The differences in moisture content of the sourdough samples could be due to differences between the two fonio seed types as well as the difference in the fermentation starter specie, as L. fermentum samples scored higher moisture content followed by L. delbrueckii and control samples (Table 2). In fact, prolonged fermentation time might increase moisture content in LAB-fermented samples compared with the naturally fermented samples (control), which was previously observed in LAB-fermented maize flour [29]. Since a lower moisture content is preferable when aiming to prolong the storability of any food product, the currently observed moisture contents were within the acceptable levels [10] with no adverse effect on the quality attribute of the product.
The protein content of the samples varied from 12.07% to 18.31%. It was observed that the samples fermented with L. fermentum had higher protein contents compared to those fermented with L. delbrueckii regardless of the fonio variety (Table 2). This could be a result of the metabolic activities and high microbial density of L. fermentum,which increased protein synthesis during fermentation [30][31]. This finding is in line with the findings of [32] who reported that lyophilized cultures of microorganisms added to cereal grains increase their basic nutritive protein quality.
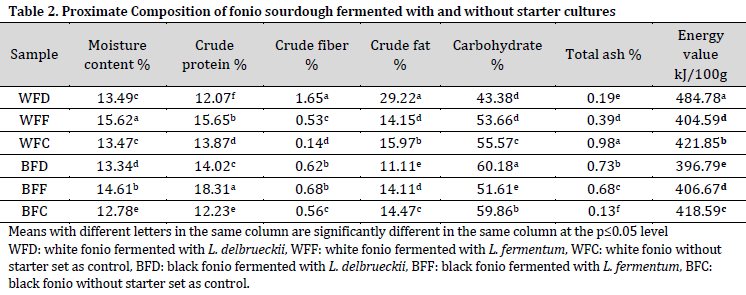
The fiber content of the samples ranged from 0.14% in WFC sample to 1.65% in WFD sample, which highlights the importance of directed fermentation in enhancing fiber content [33]. The fat content of the samples ranged from 11.11% in BFD to 29.22% in WFD. Overall, white fonio had a higher fat content when compared to black fonio, which is in agreement with previous reports [34]. The highest carbohydrate content was observed in BFD sample (60.18%) which might be attributed to the low microbial metabolic activity of L. delbrueckii in utilizing the carbohydrate in black fonio [35]. On the other hand, the use of L. fermentum and L. delbrueckii decreased the carbohydrate content of white fonio, which might refer to white fonio carbohydrates being more available for fermentation.
The ash content of the samples ranged from 0.1% to 0.98%. However, a previous report showed similar ash levels in white and black fonio [34]. Therefore, the observed differences in the ash content could be merely attributed to the differences in other compositions between the studied doughs. As for energy value, there was no significant difference in samples BFC and BFD, while WFD scored the highest (484.78 kJ/100g) among tested doughs.
Anti-nutritional factors of fonio sourdoughs
It was observed that the phytate content of sample BFF (2.74 mg/g) was significantly lower than that of the control (BFC) which scored 5.76 mg/g. This observation was similar to the findings reported by[35]that the phytic acid of fermented pearl millet was reduced from 858.4 mg/ 100 g in the raw pearl millet to 380.3 mg/100 g during the development of bread. In fact, fermentation and heat treatment are known to reduce phytate content [36]. However, this effect was not observed in WFD, WFF, or WFC, when compared to the control. Therefore, flour type and fermentation starter can both attribute to the reduction of phytate during fermentation.
Overall, oxalate, tannin, and saponin contents were lower in black fonio doughs when compared to white fonio doughs. In fact, saponin content in white fonio doughs was 5-10 times higher than their black fonio counterparts (Table 3). This means that fonio type was the determinant factor in the anti-nutritional substances content of the tested doughs.
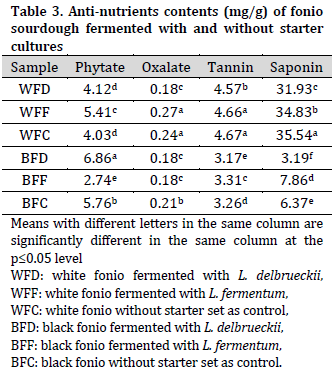
Mineral compositions of fonio sourdoughs
Overall, white fonio samples had higher iron (Fe), sodium (Na), and calcium (Ca) contents, while black fonio samples had higher potassium (K) content. Therefore, Na/K ratio was higher in white fonio samples compared to black fonio. On the other hand, similar zinc (Zn) levels were observed across all the samples (Table 4).
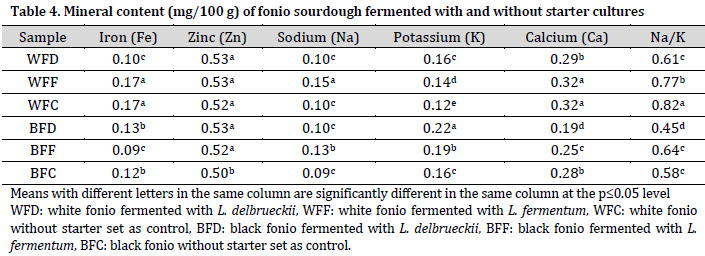
The recommended daily allowance (RDA) for Fe is 10 mg [37]. This implies that fonio flour is a poor source of iron compared to other flours, such as sorghum and soy [38]. On the other hand, the currently observed sodium levels are higher than those reported by [39].
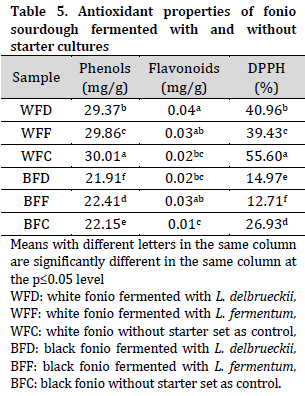
Antioxidant properties of fonio sourdoughs
Overall, higher phenol content and DPPH levels (lower free radical scavenging ability) were observed in white fonio when compared to their black fonio counterparts. While no major differences were observed in flavonoid contents between the two types (Table 5). It was noted that fonio doughs fermented with starter LAB (except for BFF) had lower phenolic content than that of control samples. This observation contradicts previous reports that phenols concentration increases as a result of LAB-mediated fermentation of cereals and pseudo-cereal flours [41-43]. However, LAB-fermented samples had a higher free radical scavenging ability (lower unscavenged DPPH%) compared to control doughs. It is also noted that L. fermentum doughs had a higher free radical scavenging ability compared to L. delbrueckii doughs. The higher free radical scavenging ability of LAB-fermented doughs highlights the antioxidant capabilities and health benefits of directed fermentation in sourdough.
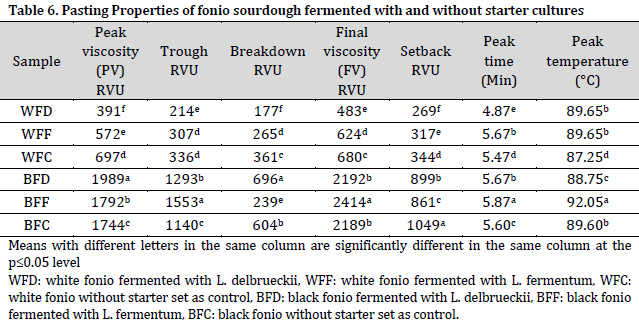
Pasting characteristics of fonio sourdoughs
The peak viscosity (PV), trough, final viscosity (FV), and setback values were higher in black fonio sourdoughs compared to those of white fonio. PV is an index for the ability of starch-based flours to swell freely before their physical breakdown [9][45]. The currently obtained PV values are higher than those reported by [44] on chinchin production from millet and wheat. Trough viscosity is the minimum viscosity value in the constant temperature phase of the RVA pasting profile which measures the ability of the paste to withstand breakdown during cooling [46]. Therefore, white fonio samples, especially WFD, would not be able to withstand breakdown during cooling due to their low low trough viscosity values, which may lead to poor product characteristics when being baked. Additionally, the low final viscosity (FV) of sample WFD could be attributed to the lower degree of re-association between the starch molecules on subsequent cooling and cooking to form a viscous paste, since FV usually measures the stability of the cooked paste [46]. On the other hand, BFF had the highest FV value which is similar to a more stable wheat and millet composite flour in a previous report [44]. Additionally, the obtained pasting temperatures are comparable to those reported by [46]. The pasting temperature is an indicator of the minimum cooking temperature of the sample. Therefore, high pasting temperature brings about high-water binding capacity and high gelatinization tendency, which normally results in low swelling properties of any starch-based flour due to the high association between starch granules [46]. This may influence the energy cost and storability of other components in the formulation, as more energy will be required to initiate starch gelatinization in flour viscosity during heating.
Conclusion
The sourdoughs of white and black fonio seeds fermented with L. delbrueckii and L. fermentum as starter cultures obtained various nutritional qualities. Therefore, LAB fermented fonio sourdoughs are of high health and nutritional importance. Further research is needed to investigate the processing of these flours in baking and to provide in-depth information regarding the acceptability and rheological behavior of these doughs.
Acknowledgments
The authors wish to acknowledge the Departments of Food Science and TechnologyLaboratories of the Federal University of Technology, Akure for the assistance offered in the processing of raw materials and running the analyses.
References
| 1 | Wang CY, Wu SJ, Shyu YT. Antioxidant properties of certain cereals as affected by food-grade bacteria fermentation. J Biosci. Bioeng. 2014 117:49–456. DOI |
| 2 | Gobbetti M, De Angelis M, Arnaut P, Tossut P, Corsetti A, Lavermicocca P. Added pentosans in breadmaking: fermentations of derived pentoses by sourdough lactic acid bacteria. Food Microbiol. 1999;16(4):409-18. DOI |
| 3 | Makanjuola SA, Enujiugha VN. How consumers estimate the size and appeal of flexible packaging. Food Qual. Pref. 2015;39:236-40. DOI |
| 4 | Poutanen K, Flander L, Katina K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009;26(7):693-9. DOI |
| 5 | Chavan RS, Chavan SR. Sourdough technology—a traditional way for wholesome foods: a review. Compr. Rev. Food Sci. Food Saf. 2011;10(3):169-82. DOI |
| 6 | Nirmala Prasadi VP, Joye IJ. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients. 2020;12(10):3045. DOI |
| 7 | Serna-Saldivar SO. CEREALS | Dietary Importance. In Encyclopedia of Food Science and Nutrition 2nd Ed. Elsevier Science BV. 2003:1027-33. DOI |
| 8 | Lobos DR, Vicuña IA, Novik V, Vega CA. Effect of high and low glycemic index breakfast on postprandial metabolic parameters and satiety in subjects with type 2 diabetes mellitus under intensive insulin therapy: Controlled clinical trial. Clin. Nutr. ESPEN. 2017;20:12 DOI |
| 9 | Fardet A, Leenhardt F, Lioger D, Scalbert A, Rémésy C. Parameters controlling the glycaemic response to breads. Nutr. Res Rev. 2006;19(1):18-25. DOI |
| 10 | National Research Council. National science education standards. National Academies Press. 1996. |
| 11 | Sanni LO, Adebowale AA, Filani TA, Oyewole OB, Westby A. Quality of flash and rotary dried fufu flour. J. Food Agric. Environ. 2006;4(3and4):74-8. |
| 12 | Enujiugha VN. Biotechnology for Healthy Nutrition and Productive Lifestyle. Federal University of Technology. 2020: 91. |
| 13 | Devi PB, Vijayabharathi R, Sathyabama S, Malleshi NG, Priyadarisini VB. Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: a review. J. Food Sci. Technol. 2014;51:1021-40. DOI |
| 14 | Guyot JP. Cereal‐based fermented foods in developing countries: ancient foods for modern research. Int. J. Food Sci. Technol. 2012;47(6):1109-14. DOI |
| 15 | Panda S, Ray R. Amylolytic lactic acid bacteria—Technological interventions in food fermentations. In Fermented Foods; Part I: Biochem & Biotechnol 1st Ed. CRC Press. 2016:133–150. |
| 16 | Xiang H, Sun-Waterhouse D, Waterhouse GI, Cui C, Ruan Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness. 2019;8(3):203-43. DOI |
| 17 | Mantziari A, Tölkkö S, Ouwehand AC, Löyttyniemi E, Isolauri E, Salminen S, Rautava S. The effect of donor human milk fortification on the adhesion of probiotics in vitro. Nutrients. 2020;12(1):182. DOI |
| 18 | Melini F, Melini V, Luziatelli F, Ficca AG, Ruzzi M. Health-promoting components in fermented foods: an up-to-date systematic review. Nutrients. 2019;11(5):1189. DOI |
| 19 | Holt JG, Krieg NR, Sneath PH, Stanley JJ. Williams ST. Bergey’s manual of determinative bacteriology. Waverly press. 1994(9):45-56. |
| 20 | Babatuyi CY, Boboye BE, Fagbemi TN, Enujiugha VN. Cyanide, haematology and histopathology profiles of albino rats fed with ‘Fufu’-based diets produced from mixed starter cultures. Heliyon. 2020;6(7):e04391. DOI |
| 21 | Ayo JA, Kajo N. Effect of soybean hulls supplementation on the quality of acha based biscuits. Am. J. Food Nutr. 2016;6(2):49-56. DOI |
| 22 | Aplevicz KS, Ogliari PJ, Sant’Anna ES. Influence of fermentation time on characteristics of sourdough bread. Brazilian J. Pharm. Sci. 2013;49:233-9. DOI |
| 23 | Horwitz W. Official methods of analysis. Washington, DC: Association of Official Analytical Chemists. 1975. |
| 24 | Gyamfi MA, Yonamine M, Aniya Y. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. General Pharmacology: The Vascular System. 1999;32(6):661-7. DOI |
| 25 | Singleton VL, Orthofer R, Lamuela-Raventós RM. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in enzymology. Academic press. 1999;299:152-78. DOI |
| 26 | Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food chem. 2005;91(3):571-7. DOI |
| 27 | Scientific N. Operation manual for the series 4 Rapid Visco Analyzer. Australia: Newport Scientific Pty, Ltd. 1995:93-5. |
| 28 | Wang D, Zhao L, Su R, Jin Y. Effects of different starter culture combinations on microbial counts and physico‐chemical properties in dry fermented mutton sausages. Food Sci. Nutr. 2019;7(6):1957-68. DOI |
| 29 | Ogodo AC, Ugbogu OC, Onyeagba RA, Okereke HC. Effect of lactic acid bacteria consortium fermentation on the proximate composition and in-vitro starch/protein digestibility of maize (Zea mays) flour. Am. J. Microbiol. Biotechnol. 2017;4(4):35-43. |
| 30 | Arte E, Rizzello CG, Verni M, Nordlund E, Katina K, Coda R. Impact of enzymatic and microbial bioprocessing on protein modification and nutritional properties of wheat bran. J. Agric. Food Chem. 2015;63(39):8685-93. DOI |
| 31 | Manini F, Brasca M, Plumed-Ferrer C, Morandi S, Erba D, Casiraghi MC. Study of the chemical changes and evolution of microbiota during sourdough like fermentation of wheat bran. Cereal Chem. 2014 91:342–9. DOI |
| 32 | El-Megeed ME, Sands DC. inventors; Development Institute Inc of Montana State University, assignee. Method and compositions for improving the nutritive value of foods via Lactobacillus ferementum. United States patent US 4,889,810. 1989. |
| 33 | Verni M, Rizzello CG, Coda R. Fermentation biotechnology applied to cereal industry by-products: Nutritional and functional insights. Front. nutr. 2019;6:42. DOI |
| 34 | Sadiq I, Maiwada SA, Dauda D, Jamilu YM, Madungurum MA. Comparative nutritional analysis of black fonio (Digitaria iburua) and white fonio (Digitaria exili). Sadiq, IZ, Maiwada, SA, Dauda, D., Jamilu, YM, & Madungurum, MA (2015). Comparative nutritional analysis of black fonio (Digitaria iburua) and white fonio (Digitaria exili). Int. J. Biol. Sci. 2015;4:4-9. |
| 35 | Ranasalva N, Visvanathan R. Development of bread from fermented pearl millet flour. J. Food Process. Technol. 2014;5(5). DOI |
| 36 | Guo J, Bian YY, Zhu KX, Guo XN, Peng W, Zhou HM. Activation of endogenous phytase and degradation of phytate in wheat bran. J. Agric. Food Chem. 2015;63(4):1082-7. DOI |
| 37 | Sandstead HH. Requirements and toxicity of essential trace elements, illustrated by zinc and copper. Am. J. Clin. Nutr. 1995;61(3):621S-4S. DOI |
| 38 | Omoba OS, Omogbemile A. Physicochemical properties of sorghum biscuits enriched with defatted soy flour. Curr. J. Appl. Sci. Technol. 2013;3(4):1246. DOI |
| 39 | Cruz JF, Beavogui F, Drame D. Le Acha, une céréale africaine. Agricultures tropicales en poche. Presses agronomiques de Gembloux. Versailles, France. 2011:175. |
| 40 | Lisinska G. The yam tuber in storage: By A. U. Osagie. Postharvest Research Unit, University of Benin, Benin City, Nigeria, 1992. 247 pp. ISBN 978-2120-00-6. Food Chem. 1994;49(4):437. DOI |
| 41 | Laurent-Babot C, Guyot JP. Should research on the nutritional potential and health benefits of fermented cereals focus more on the general health status of populations in developing countries? Microorganisms. 2017;5(3):40. DOI |
| 42 | Rizzello CG, Cassone A, Di Cagno R, Gobbetti M. Synthesis of angiotensin I-converting enzyme (ACE)-inhibitory peptides and γ-aminobutyric acid (GABA) during sourdough fermentation by selected lactic acid bacteria. J. Agric. Food Chem. 2008;56(16):6936-43. DOI |
| 43 | Rizzello CG, Nionelli L, Coda R, Gobbetti M. Synthesis of the cancer preventive peptide lunasin by lactic acid bacteria during sourdough fermentation. Nutr. Cancer. 2012;64(1):111-20. DOI |
| 44 | Adegunwa MO, Ganiyu AA, Bakare HA, Adebowale AA. Quality evaluation of composite millet-wheat Chinchin. Agric. Biol. J. North Am. 2014;5(1):33-9. |
| 45 | Adebowale AA, Sanni LO, Onitilo MO. Chemical composition and pasting properties of tapioca grits from different cassava varieties and roasting methods. Afr. J. Food Sci. 2008;2:77-82. |
| 46 | Adebowale AA, Adegoke MT, Sanni SA, Adegunwa MO, Fetuga GO. Functional properties and biscuit making potentials of sorghum-wheat flour composite. Am. J. Food Technol. 2012;7(6):372-9. DOI |
Cite this article:
Babatuyi, C., Adisa, A., I., T., Enujiugha, V. The variations in chemical composition, antioxidant capacity, and pasting properties of Fonio sourdoughs. DYSONA – Applied Science, 2023;4(2): 51-61. doi: 10.30493/das.2023.369327
