Alphonsus O. Ogbuabor 1*
1, Department of Medical Laboratory Sciences, Faculty of Basic Medical Sciences, College of Medicine, Enugu State University of Science and Technology, Enugu, Nigeria
E-mail:
ogbuaborao@yahoo.com
Received: 12/01/2024
Acceptance: 05/02/2024
Available Online: 06/02/2024
Published: 01/04/2024

Manuscript link
http://dx.doi.org/10.30493/DLS.2024.435290
Abstract
Chronic kidney disease (CKD) is a substantial worldwide health challenge, especially in low- and middle-income nations such as Nigeria. Anemia frequently occurs as a consequence of CKD, leading to higher rates of morbidity and mortality. This study examined the association between serum levels of tumor necrosis factor alpha (TNFα) and various hematological markers in patients with CKD. A cohort of 100 individuals was enlisted, consisting of 50 patients in G4 and G5 stage CKD (25 patients from each stage) and 50 healthy individuals. CKD patients exhibited notable reductions in hematological indicators, including red blood cell count, hemoglobin, mean cell volume, mean cell hemoglobin, mean cell hemoglobin concentration, and hematocrit, when compared to controls. Additionally, elevated levels of TNFα, reticulocyte, erythropoietin, and creatinine were observed in CKD patients. An evident inverse correlation was observed between TNFα levels and hemoglobin, indicating a link between elevated TNFα and decreased erythropoiesis. Moreover, a correlation between elevated creatinine levels suggests that TNFα plays a role in the decline of renal function in the studied population.
Keywords: Chronic kidney disease, Anemia, TNFα, Nigeria
Introduction
Chronic kidney disease (CKD) is a significant worldwide health issue, identified by the Global Burden of Disease (GBD) group as the 19th most common cause of illness and death. It is particularly prevalent in low- and middle-income countries like Nigeria, where it places a substantial economic burden on families [1][2]. Anemia has been recognized as a significant contributor to the high incidence of illness and low quality of life in patients with chronic renal disease, as well as an independent factor that increases the risk of death [3-6]. The prevalence of anemia in CKD patients is estimated to be 15% in the United States, 45-55% in Asian patients, and 50-90% in African patients, according to studies [7-12]. Anemia of chronic kidney disease is distinguished by a decrease in the generation of red blood cells, their lifespan, and/or the normal functioning of iron metabolism due to inflammation [13-16].
Necroptosis is an inflammatory form of programmed cell death that is regulated by Tumor Necrosis Factor Alpha (TNFα). This factor has been recognized as a significant route contributing to the development of various clinical illnesses [17]. The process entails the creation of a necrosome, which is a complex composed of specific proteins, including receptor-interacting protein kinases 1 and 3 (RIPK 1 and RIPK 3), as well as mixed lineage kinase domain-like protein (MLKL). These proteins are activated by tumor necrosis factor alpha [18]. TNF-α is a crucial multifunctional pro-inflammatory cytokine that is strongly linked to chronic inflammatory disorders [15][19]. Various studies have documented the correlation between tumor necrosis factor alpha and indicators of anemia in certain conditions. Only a limited number of research have been conducted on individuals with chronic renal disease in Nigerians. This study examined the correlation between serum levels of TNF-α and red blood cell parameters in individuals diagnosed with chronic kidney disease.
Materials and Methods
Study area
The research was conducted at the Enugu State University of Science and Technology (ESUT) Teaching Hospital, located in Enugu, Nigeria. The ESUT Teaching Hospital serves as the primary tertiary healthcare institution for the State and is conveniently situated in the heart of the capital city, Enugu, to ensure convenient access for inhabitants. Enugu State comprises three senatorial zones: Enugu East, Enugu West, and Enugu North. The senatorial zones are partitioned into seventeen local government areas, encompassing a total of 450 villages. The state acquired its name from the appellation of its capital and most populous city, Enugu. The region spans an area of 7,161 square kilometers and is inhabited by a population of approximately 3,3 million. It is located between longitudes 6°30’E and 6°55’E, and latitudes 5°15’N and 5°15’E. The population primarily consists of the Igbo ethnic group, who predominantly reside in the rural regions of South Eastern Nigeria [20].
Sample size
A minimum sample size that was representative of the population was determined by the relation [21].
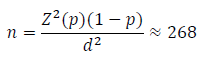
Where:
n = the sample size for a definite population
Z = 1.96 critical value at the level of significance (95% confidence interval)
p = 77.5% prevalence of anemia in chronic kidney disease patients in Enugu [11].
d = 0.05 (tolerable error)
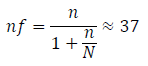
Where:
nf = minimum sample size
N = 43, which is the number of CKD patients in the registry of the Nephrology clinic in ESUT.
To increase accuracy, sample size was increased to 50 participants in each cohort. Therefore, a total of 100 subjects comprising 50 cases of chronic kidney disease patients and 50 apparently healthy control individuals were recruited for the study.
Ethical considerations
The study received ethical approval from the Research Ethical Review committee of the Enugu State University of Science and Technology (ESUT) Teaching Hospital, Enugu Nigeria, with registration number: ESUT NP/C-MAC/RA/034/Vol.4/347. Prior to enrolling participants in the study, the purpose and goals of the study were thoroughly clarified to the subjects.
Inclusion and exclusion criteria
Inclusion criteria
- Patients newly diagnosed with chronic kidney disease and were 18 years of age or older. These individuals must also have had anemia, as defined by the KDOQ1 criteria [22].
- CKD patients who have not experienced any hemorrhagic episodes in the two weeks prior to the start of the study (the minimum time required for the body’s systems to return to normal).
- CKD patients who do not have cancer or a diagnosed hematological illness.
- CKD patients who provided informed consent after receiving comprehensive information regarding the nature and objectives of the present investigation.
Exclusion criteria
- CKD patients who were undergoing dialysis and/or have previously had erythropoietin or iron replacement therapy.
- CKD patients who have received a blood transfusion within the past four weeks. It is important to note that the typical lifespan of transfused blood in a recipient is approximately 21 days.
- CKD patients who are using angiotensin converting enzyme inhibitors, particularly enalapril.
- CKD patients who declined to provide informed consent to participate in the study despite receiving comprehensive information about the purpose and objectives of the current research.
Study design
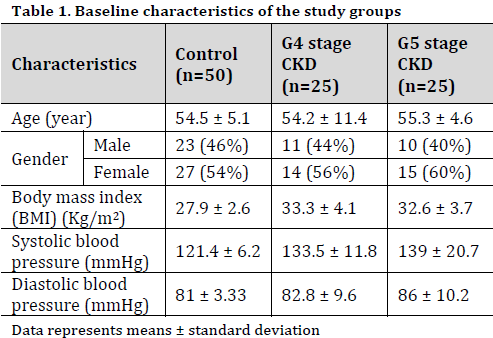
This study was conducted as a cross-sectional case-control study on patients who visited the Nephrology clinic or were hospitalized to the clinic between April and October 2023. The participants were categorized into two groups: one consisting of 50 healthy individuals, with an estimated glomerular filtration rate (eGFR) >90 ml/min/1.73m2, and the other consisting of 50 CKD patients. The CKD group was subdivided into two subgroups: CKD G4 stage (eGFR between 15 and 30 ml/min/1.73m2) subgroup, consisting of 25 participants, and CKD G4 stage (eGFR<15 ml/min/1.73m2) subgroup, also comprising of 25 patients (Table 1).
Blood sample collection
Aseptically, ten milliliters of blood were extracted through venipuncture. Five milliliters were placed in an EDTA bottle to assess red blood cell parameters, while the remaining five milliliters were placed in plain bottles, centrifuged, and stored frozen for the assessment of tumor necrosis factor alpha levels.
Measurement of blood and red blood cell parameters
The blood and red blood cell parameters, including red blood cell count, hemoglobin level, hematocrit percentage, mean cell volume level, mean cell hemoglobin level, mean cell hemoglobin concentration, reticulocyte percentage, erythropoietin level, and creatinine level, were measured using the 5-parts automated hematology analyzer (Mindray bc-6800, Shenzhen, China).
Measurement of serum TNFα
The levels of serum tumor necrosis factor alpha were measured using the Sandwich Enzyme-Linked Immunosorbent Assay (ELISA) technique. A kit from Sunlong Diagnostic Ltd (Catalog Number: AH 5278F, China) was used, and the absorbance was read at 450nm using a Microplate Reader (Mindray 96A, Shenzhen China).
Data analysis
The data was analyzed using GraphPad Prism version 8.0 and the results were provided as mean±standard deviation (SD). One-Way Analysis of Variance (ANOVA) was employed to analyze the data and Tukey’s test at 0.05 level was used to compare the means. Pearson correlation test was utilized to ascertain the association between serum TNFα levels and the observed blood parameters.
Results
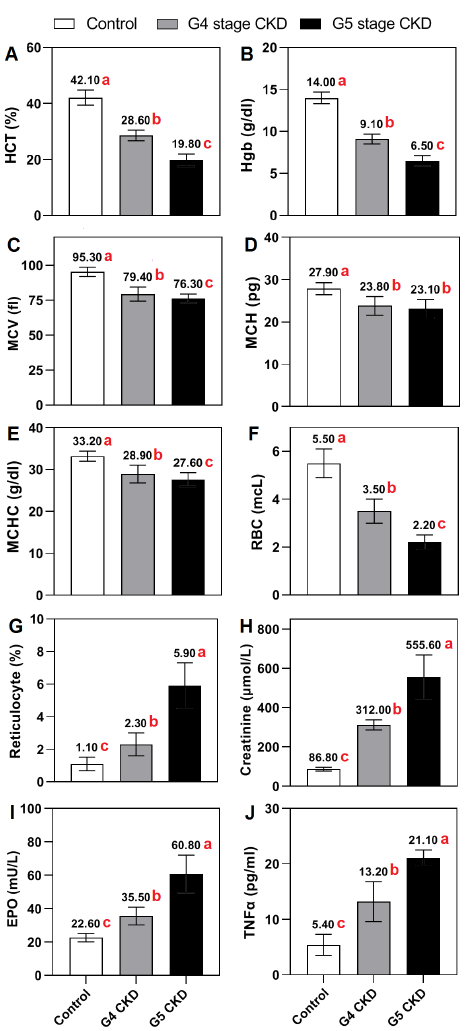
Significant variations in hematological markers were noted among the groups. The hematocrit levels were significantly decreased in both the stage G4 (28.6 ± 1.9%) and stage G5 CKD groups (19.8 ± 2.2%) compared to the healthy control group (42.1 ± 2.7%) (Fig. 1 A). Similarly, the levels of hemoglobin exhibited a significant decline in the stage G4 (9.1 ± 0.6 g/dl) and stage G5 CKD groups (6.5 ± 0.6 g/dl) as compared to the control group (14.0 ± 0.7 g/dl) (Fig. 1 B). Mean Cell Volume (MCV), Mean Cell Hemoglobin (MCH), and Mean Cell Hemoglobin Concentration (MCHC) showed substantial decreases in both CKD stages compared to the control group (Fig. 1 C-E).
The CKD groups showed a considerable drop in red blood cell (RBC) counts, with noticeable disparities detected between all groups (Fig. 1 F). The G5 CKD group had a significantly increased reticulocyte percentage compared to both the G4 CKD group and the control group (Fig. 1 G).
The levels of creatinine were considerably higher in the stage G4 (312 ± 26.2 µmol/L) and stage G5 CKD groups (555.6 ± 113 µmol/L) compared to the control group (86.8 ± 9.4 µmol/L) (Fig. 1 H). Elevated levels of erythropoietin were identified in the CKD groups compared to the control group, with the greatest levels found in stage G5 (60.8 ±11.3mU/L) (Fig. 1 I).
The levels of TNFα were significantly higher in both stage G4 (13.2 ± 3.6 pg/ml) and stage G5 (21.1 ± 1.4 pg/ml) CKD groups compared to the healthy control group (5.4 ± 1.9 pg/ml) (Fig. 1 J). All indicators, except for MCH, showed significant differences between the two CKD groups. MCH did not reveal any significant difference between the G4 and G5 CKD groups.
Pearson correlation analysis showed that the levels of TNFα was significantly and negatively correlated with the count of red blood cells, hematocrit, hemoglobin, MCV, and MCH, suggesting a connection between increased TNFα and reduced erythropoiesis. Moreover, there was a positive relationship observed between TNFα and creatinine levels, indicating a possible link between TNFα and the deterioration of renal function (Table 2).
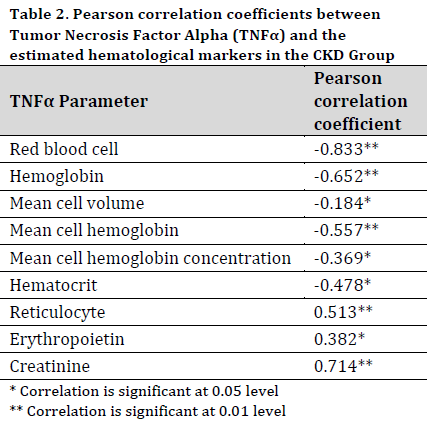
Discussion
The present investigation examined the levels of tumor necrosis factor alpha (TNFα) and anemia markers in patients with chronic kidney disease and healthy individuals. The objective was to discover if there is a significant correlation between TNFα and the development of anemia in chronic kidney disease. An evident rise in TNFα, reticulocyte count, creatinine levels, and erythropoietin (EPO) levels was noted as CDK stage progressed. In contrast, CDK patients exhibited a significant drop in hemoglobin, hematocrit, red blood cell count, mean cell volume, mean cell hemoglobin, and mean cell hemoglobin concentrations, as compared to the healthy cohort. This aligns with the results of previous research, which have indicated that anemia of CDK is linked to an increase in TNFα expression in patients with chronic kidney disease compared to control subjects [23].
Previous studies have indicated a higher prevalence of anemia in patients with stage G4 and G5 CKD [24]. The results presented in this study showed that the average hemoglobin levels in individuals with G4 and G5 CKD were measured at 9.1 and 6.5 g/dl, indicating moderate and severe anemia correspondingly [25]. Hence, a significant association was seen between the progression of CKD and the occurrence of anemia in individuals with advanced CKD in the examined group.
The levels of TNFα exhibited a negative connection with the count of red blood cells, hemoglobin levels, mean cell volume, mean cell hemoglobin, mean cell hemoglobin concentration, and hematocrit. This is consistent with the results of other investigations that have also observed a negative connection between serum TNFα levels and hemoglobin levels in cases of anemia associated with B-cell chronic lymphocytic leukemia [26] and malaria [27]. The reactivity of erythrocyte precursor cells to EPO has also been reported to have an inverse correlation with the levels of circulating TNFα in the bloodstream [28]. The elevated TNFα levels appear to be the cause of the impairment in red blood cell formation and the increased destruction of red blood cells by programmed cell death, as a result of inflammation, in patients with chronic kidney disease [29]. It is important to mention that anti-TNFα medication has been found to improve anemia in chronic kidney disease [30]. Therefore, the present study is not one of the first to demonstrate a connection between serum TNFα and anemia, specifically in patients with CKD-associated anemia. However, the findings described here further underscore the significance of the TNFα pathway in the progression of anemia in CKD patients.
Conclusion
This study presents evidence of a strong link between high levels of serum tumor necrosis factor alpha (TNFα) and impaired red blood cell properties in patients with chronic kidney disease (CKD). The results indicate that inflammation caused by TNFα may have a significant impact on the development of anemia in CKD. Additional investigation into the mechanisms that cause TNFα-induced anemia and the possibility of targeting TNFα signaling pathways for therapeutic purposes could provide innovative strategies for controlling anemia in CKD patients, especially in resource-constrained settings such as Nigeria.
Acknowledgement
Special thanks to my colleagues at the Hematology Laboratory of the Enugu State University of Science and Technology Teaching Hospital, Enugu, Nigeria for their support in sample collection during the study.
References
| 1 | Chukwuonye II, Ogah OS, Anyabolu EN, Ohagwu KA, Nwabuko OC, Onwuchekwa U, Chukwuonye ME, Obi EC, Oviasu E. Prevalence of chronic kidney disease in Nigeria: systematic review of population-based studies. Int. J. Nephrol. Renov. Dis. 2018;11:165–72. DOI |
| 2 | Olanrewaju TO, Aderibigbe A, Popoola AA, Braimoh KT, Buhari MO, Adedoyin OT, Kuranga SA, Biliaminu SA, Chijioke A, Ajape AA, Grobbee DE. Prevalence of chronic kidney disease and risk factors in North-Central Nigeria: a population-based survey. BMC Nephrol. 2020;21. DOI |
| 3 | Raji YR, Ajayi SO, Akingbola TS, Adebiyi AO, Adedapo KS, Salako BL. Diagnostic performance of peripheral blood film and red blood cell indices as markers of iron deficiency among patients with chronic kidney disease in low resource settings. Pan Afr. Med. J. Clin. Med. 2020;3(180). DOI |
| 4 | Asaduzzaman M, Shobnam A, Farukuzzaman MD, Gaffar A, Juliana FM, Sharker T, Dutta KK, Islam MJ. Assessment of Red Blood Cell Indices, White Blood Cells, Platelet Indices and Procalcitonin of Chronic Kidney Disease Patients under Hemodialysis. Int. J. Health Sci. Res. 2018;8:98-109. |
| 5 | Iyawe I, Adejumo O. Hematological profile of predialysis chronic kidney disease patients in a tertiary hospital in Southern Nigeria. J. Med. Trop. 2018;20(1):36. DOI |
| 6 | Bissinger R, Nemkov T, D’Alessandro A, Grau M, Dietz T, Bohnert BN, Essigke D, Wörn M, Schaefer L, Xiao M, Beirne JM. Proteinuric chronic kidney disease is associated with altered red blood cell lifespan, deformability and metabolism. Kidney Int. 2021;100(6):1227-39. DOI |
| 7 | Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9(1):e84943. DOI |
| 8 | Ryu SR, Park SK, Jung JY, Kim YH, Oh YK, Yoo TH, Sung S. The prevalence and management of anemia in chronic kidney disease patients: result from the KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD). J. Korean Med. Sci. 2017;32(2):249–56. DOI |
| 9 | Maina CK, Karimi PN, Mariita K, Nyamu DG, Mugendi GA, Opanga SA. Correlates and management of anemia of chronic kidney disease in a Kenyan Tertiary hospita. East Afri. Med. J. 2016;93(10):489-99. |
| 10 | Amoako YA, Laryea DO, Beddu-Addo G, Andoh H, Awuku YA. Clinical and demographic characteristics of chronic kidney disease patients in a tertiary facility in Ghana. Pan Afric. Med. J. 2014;18:274-84. DOI |
| 11 | Ijoma C, Ulasi I, Ijoma U, Ifebunandu N. High prevalence of anemia in predialysis patients in Enugu, Nigeria. Nephrol. Res. Rev. 2010;2(1):61-5. DOI |
| 12 | Adera H, Hailu W, Adane A, Tadesse A. Prevalence Of Anemia And Its Associated Factors Among Chronic Kidney Disease Patients At University Of Gondar Hospital, Northwest Ethiopia: A Hospital-Based Cross Sectional Study. Int. J. Nephrol. Renovasc. Dis. 2019;12:219-28. DOI |
| 13 | Raad Y. Hematological parameter of the blood count in patients undergoing hemodialysis. Technium Bio. Chem. Med. 2021;2(1):32-40. |
| 14 | Ganz T. Anemia of Inflammation. N. Engl. J. Med. 2019;381(12):1148-57. DOI |
| 15 | Madu AJ, Ughasoro MD. Anaemia of Chronic Disease: An In-Depth Review. Med. Princ. Pract. 2017;26(1):1-9. DOI |
| 16 | Lee YG, Chang Y, Kang J, Koo DH, Lee SS, Ryu S, Oh S. Risk factors for incident anemia of chronic diseases: A cohort study. PLoS One. 2019;14(5):e0216062. DOI |
| 17 | Yang Y, Li X, Zhang T, Xu D. RIP kinases and necroptosis in aging and aging-related diseases. Life Med. 2022;1(1):2-20. DOI |
| 18 | Ye K, Chen Z, Xu Y. The double-edged functions of necroptosis. Cell Death Dis. 2023;14(2):1–12. DOI |
| 19 | Mathanraj S, Kumar V, Yuvarajan S, Reddy V. Correlation of serum TNF alpha level with severity of chronic obstructive pulmonary disease. Int. J. Res. Med. Sci. 2017;5(8):3309-16. DOI |
| 20 | Ogbuabor AO, Juliet CA, Anayo US. Significance of Some Haemostasis Parameters in Type 2 Diabetic Patients in the Enugu State University of Science and Technology Teaching Hospital Enugu State Nigeria. Int. J. Res. Rep. Hematol. 2022;5(2):237–42. |
| 21 | Achigbu KI, Odetunde OI, Chinawa JM, Achigbu EO, Ikefuna AN, Emodi IJ, Ibe BC. Pulmonary function indices in children with sickle cell anemia in Enugu, south-east Nigeria. Saudi Med. J. 2015;36(8):928-34. DOI |
| 22 | Cases A, Egocheaga MI, Tranche S, Pallarés V, Ojeda R, Górriz JL, Portolés JM. Anemia of chronic kidney disease: Protocol of study, management and referral to Nephrology. Nefrología (English Edition). 2018;38(1):8-12. DOI |
| 23 | Keithi-Reddy SR, Addabbo F, Patel TV, Mittal BV, Goligorsky MS, Singh AK. Association of anemia and erythropoiesis stimulating agents with inflammatory biomarkers in chronic kidney disease. Kidney Int. 2008;74(6):782. DOI |
| 24 | Kimura T, Snijder R, Nozaki K. Diagnosis patterns of CKD and anemia in the Japanese population. Kidney Int. Rep. 2020;5(5):694-705. DOI |
| 25 | Badireddy M, Baradhi KM. Chronic anemia. StatPearls Publishing: Treasure Island, FL, USA. 2018. (Accessed on 2 February 2024) Link |
| 26 | Capalbo S, Battista C, Delia M, Ciancio A, De Santis G, Dargenio M, Diomede D, Liso V. Evaluation of tumor necrosis factor-alpha and erythropoietin serum levels in B-cell chronic lymphocytic leukemia patients with anemia. Acta Haematol. 2002;108(2):84-9. DOI |
| 27 | Sarangi A, Mohapatra PC, Dalai RK. Serum cytokine TNF-alpha and hemoglobin levels in Plasmodium falciparum malaria – A correlative study in coastal districts of Odisha. Apollo Med. 2012;9(4):292-6. DOI |
| 28 | de Oliveira Júnior WV, Sabino A de P, Figueiredo RC, Rios DRA. Inflammation and poor response to treatment with erythropoietin in chronic kidney disease. J. Bras. Nefrol. 2015;37(2):255-63. DOI |
| 29 | Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J. Interferon Cytokine Res. 2009;18(8):555-9. DOI |
| 30 | Shu W, Pang Z, Xu C, Lin J, Li G, Wu W, Sun S, Li J, Li X, Liu Z. Anti-TNF-α monoclonal antibody therapy improves anemia through downregulating hepatocyte hepcidin expression in inflammatory bowel disease. Mediators Inflamm. 2019; 4038619. DOI |
Cite this article:
Ogbuabor, A. Tumor necrosis factor alpha and anemia in chronic kidney disease patients: Insights from a Nigerian case-control study. DYSONA – Life Science, 2024;5(1): 29-36. doi: 10.30493/dls.2024.435290
