Ismail Muhammad 1*; Bala Abubakar 2; Mahmoud T. Mohammed 3; Aishatu Abdullahi 1; Asiya M. Usman 4; Sulaiman Abubakar 5
1, Zoology Department, Gombe State University, Gombe, Nigeria
2, Zoology Department, Modibbo Adama University, Adamawa State, Yola, Nigeria
3, Department of Biomedical and Pharmaceutical Technology, Federal Polytechnic, Mubi, Adamawa State, Nigeria
4, Preliminary and general Studies department, Federal College of Horticulture Dadin Kowa, Gombe State, Nigeria
5, Department of Microbiology, Federal University Dustin-Ma, Kastina State, Nigeria
E-mail:
muhammadismail5609@gsu.edu.ng
Received: 20/05/2022
Acceptance: 18/06/2022
Available Online: 19/06/2022
Published: 01/10/2022

Manuscript link
http://dx.doi.org/10.30493/DLS.2022.343222
Abstract
Malaria is a life-threatening parasitic disease. Successful treatment and management of malaria mainly depend on the proper and effective diagnosis. Therefore, this paper aimed to evaluate the effectiveness of a Conventional Rapid Diagnostic Test (cRDT) in comparison to microscopy. Blood samples (2-3 ml) were collected from 200 consented study subjects (Volunteers) and analyzed using microscopy and cRDT. The result revealed an overall prevalence of 139 (69.5%) and 126 (63.8%) by microscopy and cRDT, respectively. The sensitivity of the cRDT was 71.94%, while the specificity value was 57.38%. The positive and negative predictive values were 79.40% and 47.30%, respectively. Therefore, cRDT shows high sensitivity and predictive values with moderate specificityand negative predictive values. Although these results are considered low compared to the recommendations of the world health organization, cRDT is recommended especially in rural communities and cities where standard laboratories are lacking, especially in emergencies.
Keywords: RDT, Malaria, Gold standard, Sensitivity, Specificity, Predictive values
Introduction
Malaria is a fatal parasitic disease that has become a complex and overwhelming public health issue, particularly in tropical countries where the environment supports the growth of both the parasite and vector [1]. The disease has been with humanity for over 5,000 years, with the first written evidence appearing in the Chinese medical classic Nei Chin (the Canon of Medicine) [2]. Over half of the world’s population lives in malaria-prone areas [3], and the illness is caused by the protozoan Plasmodium species falciparum, vivax, malariae, and ovale,withthe latter having two subspecies, curtisi, and wallikeri. Recently, new Plasmodium species have been microscopically identified (i.e. P. knowlesi, cynomolgi and simium), all zoonosis transmitted, the first two, by macaques in Southeast Asia, while P. simium by monkeys from the Brazilian Atlantic forest. However, P. falciparum and P. vivax are the most common species causing the disease, and of these falciparum is the most lethal, as it causes malignant or cerebral malaria, which can quickly progress to unconsciousness and death due to infected red cell adhesion to the vascular endothelium (cytoadherence) [4]. It is also common to observe “mixed” infections, with the simultaneous presence of Plasmodium of different species in endemic areas.
In 2016, an estimated 216 million cases of malaria were reported worldwide, with 445,000 deaths [5], where about 90% of the global malaria cases are recorded among children under five years and pregnant women. Malaria is endemic throughout Nigeria [6], where it is responsible for about 60% of outpatient visits to health facilities, 30% of childhood mortality, 25% of deaths of children under one year, and 11% of maternal mortality. Plasmodium falciparum accounts for 98% of malaria mortalities in Nigeria [7]
World Health Organization strongly opposes the blind treatment of malaria and highly recommends an evidence-based treatment that demonstrates the presence of parasites or parasite parts/products in the body and their developmental stages [8][9], which is only achievable through early and effective diagnosis [10]. In fact, antimalarial drugs are frequently misused because of the lack of a proper diagnosis of the disease before treatment. As a result, the parasite can develop resistance through drug pressure [3], which has economic implications and can also put the individual at risk. Therefore, accurate diagnosis is critical in reducing morbidity and mortality and long-term planning for disease elimination in endemic areas [11]. As recommended by the World Health Organisation, the primary tool for the control of the disease in many parts of Africa remains early diagnosis and treatment of clinical cases [12], combined with prompt, effective, and well-tolerated treatment through the use of recommended antimalarial drugs in disease management [13] and gametocytocidal drug targets therapy, as there is no suitable vaccine treatment for the disease [14]. As a result, successful malaria prevention, treatment, and control are inextricably linked to an accurate diagnosis. Diagnostic tools in Plasmodium research follow precise steps starting with thin and thick blood smears (TnS, TkS), in addition to quantitative buffy coat (QBC), loop-mediated isothermal amplification (LAMP), rapid diagnostic tests (RDTs), and lateral flow assay (LFA) employing antibodies in single or in various combination against P. falciparum-specific histidine-rich protein 2 (HRP-2) antigen, P. vivax-specific lactate dehydrogenase (LDH) antigen, pan-Plasmodium LDH antigen, and finally, Plasmodium genus qPCR as a reference standard.
Despite low sensitivity, the TnS/TkS are accepted as a gold standard diagnostic method for detecting, identifying, and quantifying species of the malaria parasite and their circulating stages (e.g., trophozoites, schizonts, gametocytes). However, this test has limitations in low parasitemia conditions such as chronic infection, early stage of disease, partially treated condition, and the loss of parasites during staining, which results in a false-negative cohort. False outcomes result in poor control of the disease, resulting in the endemicity of the disease [15]
Microscopy is the best standard technique for malaria diagnosis with a high level of accuracy compared to other methods, mainly when carried out by well-trained and experienced laboratory technicians. As a result, the technologist’s skill and experience play a crucial role in the reliability of microscopy results [16]. Microscopy has several advantages, including distinguishing between parasite species and developmental stages, observing morphological changes caused by recent treatment, and quantifying and storing samples for long periods, allowing for later quality control [17]. However, human resources, electricity, essential equipment, and reagents are some of the “bottlenecks” associated with microscopy, particularly in rural areas and developing countries [18][19], coupled with the fact that it takes time to perform and is labor-intensive [20]. The technique also cannot detect sequestered P. falciparum parasites on the peripheral blood film, and it is less sensitive when the parasite density is less than 50 parasites per microliter of blood [21][22]. Apart from these factors, most malaria cases occur in rural areas where there is limited or no access to reference laboratories, and in many cases, microscopy is not available [23], hence, necessitating the use of alternative diagnostic tests such as conventional rapid diagnostic tests.
Rapid diagnostic tests (RDTs) have the potential to provide accurate diagnoses to all at-risk populations, including those who do not have access to microscopy services in endemic areas. These tests look for specific malaria parasite antigens in patients’ blood. HRP – 2 (Histidine-rich protein 2), PLDH (Parasite Lactate Dehydrogenase), and Plasmodium Aldolase (a combination of PLDH and HRP -2) are some of the antigens involved [24][25]. Each technique has its own set of advantages and disadvantages with regard to sensitivity, specificity, time consumption, cost-effectiveness, and procedure ease [26]. As a result, all of these and other novel diagnostic techniques, whether molecular, serological, or proteomic, must be compared to microscopy, which is still considered the gold standard, to be considered reliable [27]. Therefore, the aim of this study was to determine the effectiveness of a conventional RDT in malaria diagnosis compared to microscopy.
Material and Methods
Study Area
The study was conducted in Gombe Local Government Area, (Fig. 1) Gombe State, Nigeria. The Local Government lies between 11°14′07″E and 11°4′42″E, and Latitudes 10°16′48″N and 10°17′24″N with a total land mass of 52km2. Gombe Local Government has a projected population figure of 367,500people (3.3% annual change). The vegetation of the local government is typical of that of Gombe state, Sudan savannah, and experiences two distinct seasons, dry season, which usually commences from November to March, and a rainy season from April to October. Agriculture is the primary occupation in the region (mostly Peasant farmers), while some engage in business and few are civil servants. As the state capital, Gombe local government has tertiary (Federal Teaching Hospital) and secondary (Gombe State Specialist Hospital) health facilities. This is also in addition to the primary health care centers strategically located in each local government ward, with several private hospitals providing different services, including malaria diagnosis and treatment.
Source: GIS Laboratory, Geography department, Gombe State University
Ethical consideration
The research proposal was submitted to Gombe State Ministry of Health for approval. Afterward, the approval was communicated with the approval MOH/ ADM/621/VOL.I/222 dated 21st February 2020.
Consent of the subjects
Before collecting blood samples from the study subjects, their verbal or written consent was sought after briefing them on the research and the need for them to participate. In a situation whereby the subjects were not mature enough, consent of their parents/guardian were sought. All the subjects were assured that all collected information was strictly used for the research and treated with confidentiality. Additionally, quality control was assured when handling and treating each sample.
Study centers
Three recruitment centers were selected: Gombe town maternity (Gidan Magani), Sunnah clinic, and Idi children and Women Hospital Gombe, where a total of 200 volunteers actively participated in the study.
Inclusion and exclusion criteria
Only patients who reported themselves to the selected hospitals with fever or history of fever in the last 24 hours and referred by a physician for the screening of malaria infection with the presumption of being malaria positive were included in the study.
Blood sample collection
The blood samples were collected with the help of medical personnel and the method employed was venepuncture techniques. The collected blood sample (2-3 ml) was transferred into an EDTA blood collecting tube and transported to the laboratory.
Rapid diagnostic test (cRDT)
A conventional rapid diagnostic test kit (SD Bio line Malaria Ag P.f (05fk50)) was used. The detecting part of this kit is a mouse monoclonal antibodies coated membrane strip. These antibodies are specific to Plasmodium falciparum HRP-II. The mouse monoclonal antibodies specific to HRP-II react with malaria Plasmodium falciparum in the specimen, moving along the membrane chromatographically to the test region and forming a visible line.
About 5μl of the collected blood sample was taken with a pipette provided with the kit. Afterward, it was loaded into the sample well (S) of the cRDT. Two drops of provided buffer were loaded into the buffer well of the same cRDT. The result was then read within 15-20 minutes as recommended by the manufacturer.
Microscopy
The collected blood samples were analyzed within 1 to 2 hours after collection. Thick and thin films (TnS/TkS) were prepared according to the standard technique. Briefly, a drop of blood sample was placed on the center of grease-free slides. After which, the reverse side of the slides was cleaned with cotton wool, allowed for air-drying, and stained with Giemsa stain for 1 hour. After which, the slides were washed off gently with clean water. The prepared slides were examined under a microscope using oil immersion at 100× magnification to observe for Plasmodium parasite. The presence of ring forms or Trophozoites of Plasmodium indicated positive results. In contrast, the absence of either Trophozoites or ring form after 10 minutes of a thorough examination by a qualified microscopist indicates a negative result.
Determination of cRDT accuracy
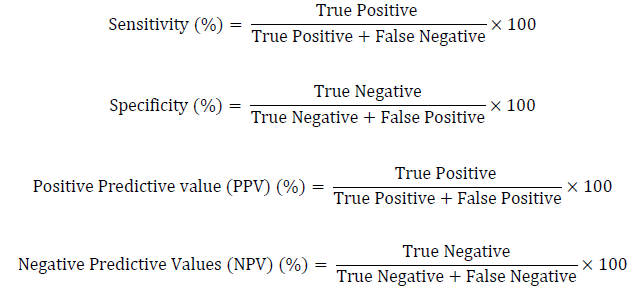
For the determination of cRDT accuracy, sensitivity, specificity, positive predictive, and negative predictive values were calculated using the formula adopted from [28]:
The collected cRDT and microscopy results was subjected to Chi-square test for comparison at P≤ 0.05.
Results
Demographic distribution of study subjects
A total of 200 study subjects, 113 (56.50%) male and 87 (43.5%) female, participated in the study. The age of the study subjects ranged from 5 to 55 years, with an average age of 28.51±10.57. The age group of 31 to 35 years was the most frequent, with 48 (24.8%) participants. The mean body temperature of the subject at the time of recruitment was 37.7±1.97.
Malaria prevalence
Out of the 200 collected blood samples, an overall prevalence of 139 (69.5%) was recorded using the gold standard techniques (microscopy). On the other hand, cRDT showed a prevalence of 126 (63.0%) (Fig. 2). A significant difference between the two techniques was observed (χ2=15.635 P<0.05).
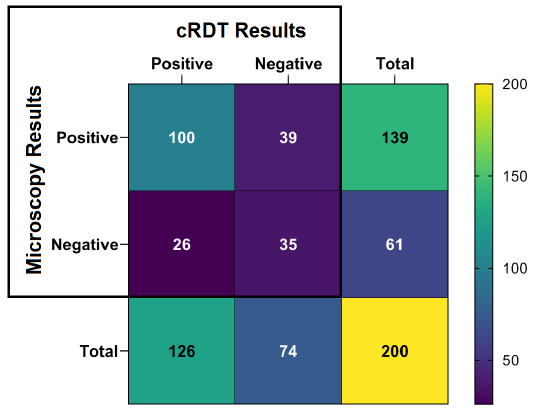
Among the investigated samples, 100 (50%) were positive in both microscopy and cRDT and considered true positive cases for cRDT. On the other hand, only 35 negative samples were confirmed by microscopy and cRDT and considered true negative (Fig. 2).
cRDT accuracy values
The sensitivity and specificity values of cRDT recorded in this research were 71.94% and 57.38%, respectively. On the other hand, the positive and negative predictive values were 79.37% and 47.30%, respectively.
Discussion
The ability to recognize subjects with a medical condition while also excluding any other subjects who do not have that illness condition is the cornerstone of any reliable diagnosis test [29]. The findings from the present study revealed an overall malaria prevalence of 69.5% and 63.0% when diagnosed using microscopy (gold standard technique) and RDT, respectively. The result is slightly similar to 64.89% by microscopy and 60.69% by RDT reported by [30] from Adamawa state Nigeria. This similarity was not surprising, as the two study areas (Gombe and Adamawa) are located in the same region with boundaries. Therefore, they share many similar predisposing factors of malaria infection that could facilitate the development and survival of both the vector and parasite of malaria. This observation also demonstrates a similar level of malaria endemicity in the region. The prevalence of malaria by microscopy recorded in this study is far higher than the 2.3% reported by [31] from Malawi among pregnant women. This difference could be attributed to the ability of malaria parasite to sequester into some vital organs, especially the placenta [32], therefore becoming absent in the peripheral blood and missing with microscopic diagnosis. Additionally, microscopy is limited by the amount of parasite in a given blood sample, while RDT depends on the presence of the parasite’s antigens [33][34].
Although the sensitivity (71.94%) recorded in this study is higher than that (23.4%) reported by [35] from Ogun state, Nigeria, the currently reported sensitivity and specificity (57.38%) are still lower than 95% and 90%, respectively, recommended by the World Health Organisation. However, the kit is reliable in detecting up to 100 parasites per microliter of blood. The currently reported RDT sensitivity and specificity below the recommended threshold could be attributed to several factors and conditions. For instance, cRDT positivity and TnS/TkS negativity (such as the reported 26 false-positive cRDT cases) in subjects treated for P. falciparum is typical due to the high concentrations of PfHRP2 antigen with the absence or low presence of Plasmodia in peripheral blood [32]. On the other hand, most commercially available RDTs, including the one reported in this study, target PfHRP2. The accuracy and reliability of this specific targetting might be jeopardized by PfHRP2 lacking isolates of P. falciparum or those having multiple variants of this gene, which might justify the 39 false-negative cases to some extent. Therefore, assessing PfHRP2 diversity in endemic regions is crucial in developing more reliable RDT.
Positive and negative predictive values of 79.37% and 47.30% were respectively recorded in the present study. These results are comparable to [35], who reported a positive predictive value of 69.1% and a negative predictive value of 43%.
In cases of total dominance of a Plasmodium species in the territory, the use of a multi-analyte RDT could be expensive. However, if the dominance favors a Plasmodium more challenging to identify with cRDT, a multi-analyte RDT choice is always preferable. In the work of [36], the highly endemic area in coastal India was investigated. 87.85% of 2016 malaria registered cases in this area were due to Plasmodium vivax, while a low percentage of falciparum was observed. The researchers used RDTs to detect three different types of Plasmodium antigen: PfHRP-2, plasmodium lactate dehydrogenase (pLDH), and plasmodium aldolase, rather than PfHRP2-only kits. Therefore, the developed MRDTs can be used to detect any malaria species, whether present alone or in a combination.
Conclusion
Low predictive values recorded in the present study are attributed to the large numbers of false-positive (26) and negative (39) cases, which directly influence predictive values. Interestingly, the positive predictive value of 79.37% implies that the false-positive effect was minimized, while the lower negative predictive value of 47.30% implies that there were too many false-negative cases. Although cRDT has demonstrated high levels of sensitivity and positive predictive value, these values, along with specificity negative predictive values, remain lower than those recommended by the World Health Organisation. However, cRDTs can still be used in rural communities and some cities in case of emergencies. In this case, cRDT results should be followed with a confirmatory test using any other available malaria diagnostic method.
References
| 1 | Achi NK, Onyeabo C, Nnate DA, Ekeleme-Egedigwe CA, Kalu IK, Chibundu IC, Wokoma GC. Therapeutic effects of Azadirachta indica A. Juss. leaves in malaria-induced male Wistar rats. J Pharm Pharmacogn Res. 2018;6(3):191-204. |
| 2 | Frey C, Traoré C, De Allegri M, Kouyaté B, Müller O. Compliance of young children with ITN protection in rural Burkina Faso. Malar. J. 2006;5(1):1-8. DOI |
| 3 | Olasehinde GI, Ojurongbe DO, Akinjogunla OJ, Egwari LO, Adeyeba AO. Prevalence of Malaria and Predisposing Factors to Antimalarial Drug Resistance in Southwestern Nigeria. Res. J. Parasitol. 2015;10(3):92–101. DOI |
| 4 | Olatunji P.O. Malaria and the Sickle Gene : Polymorphism Balance in favour of eradication. Ann Heal Res. 2018;4(2):88–96. DOI |
| 5 | Gontie GB, Wolde HF, Baraki AG. Prevalence and associated factors of malaria among pregnant women in Sherkole district , Benishangul Gumuz regional state , West Ethiopia. BMC Infect Dis. 2020;20(573). DOI |
| 6 | Urindwanayo D, Ogbuagu CN, Ekwunife CA. The prevalence of malaria infection among secondary school students in Oba Idemili South Local Government Area,Anambara State Nigeria. Texila Int. J. Public Health. 2015;3(2). |
| 7 | Omole MK, Onademuren OT. A survey of antimalarial drug use practices among urban dwellers in Abeokuta, Nigeria. Afr J Biomed Res. 2010;13(1). |
| 8 | Omalu IC, Uzoaga G, Olayemi IK, Mgbemena C, Hassan S, Pam V, Lateef A, Eke SS. Evaluating the use of microscopic examination and rapid diagnostic tests to diagnose malaria in North Central Nigeria. Malar Rep. 2014;3(1836):5–7. DOI |
| 9 | Nantavisai K. Malaria detection using non-blood samples. Songklanakarin J Sci Technol. 2014;36(6):633–41. |
| 10 | Jangale N, Waghmare A. Acridine orange for diagnosis of malaria – Our experience. South East Asia J Public Health. 2016;6(1):49–51. DOI |
| 11 | Moyeh MN, Ali IM, Njimoh DL, Nji AM, Netongo PM, Evehe MS, Atogho-Tiedeu B, Ghogomu SM, Mbacham WF. Comparison of the accuracy of four malaria diagnostic methods in a high transmission setting in coastal Cameroon. J Parasitol Res. 2019. DOI |
| 12 | Mbacham WF, Evehe MS, Netongo PM, Ateh IA, Mimche PN, Ajua A, Nji AM, Irenee D, Echouffo-Tcheugui JB, Tawe B, Hallett R. Efficacy of amodiaquine, sulphadoxine-pyrimethamine and their combination for the treatment of uncomplicated Plasmodium falciparum malaria in children in Cameroon at the time of policy change to artemisinin-based combination therapy. Malar J. 2010;9(1). DOI |
| 13 | Pelfrene E, Pinheiro MH, Cavaleri M. Artemisinin-based combination therapy in the treatment of uncomplicated malaria: review of recent regulatory experience at the European Medicines Agency. Int Health. 2015;7(4):239-46. DOI |
| 14 | Sumsakul W, Plengsuriyakarn T, Chaijaroenkul W, Viyanant V, Karbwang J, Na-Bangchang K. Antimalarial activity of plumbagin in vitro and in animal models. BMC Complement Altern Med. 2014;14(1). DOI |
| 15 | Arshad AR. Thrombocytopenia in malaria: can platelet counts differentiate malaria from other infections. J Coll Physicians Surg Pak. 2015;25(1):31-4. |
| 16 | Hathiwala R, Mehta PR, Nataraj G, Hathiwala S. LED fluorescence microscopy: Novel method for malaria diagnosis compared with routine methods. J. Infect. Public Health. 2017;10(6):824-8. DOI |
| 17 | Di Santi SM, Kirchgatter K, Brunialti KC, Oliveira AM, Ferreira SR, Boulos M. PCR – Based diagnosis to evaluate the performance of Malaria referrence centres. Rev. Inst. Med. Trop. Sao Paulo. 2004;46(4):183–7. DOI |
| 18 | Kudisthalert W, Pasupa K, Tongsima S. Counting and Classification of Malarial Parasite From Giemsa-Stained Thin Film Images. IEEE Access. 2020;8:78663–82. DOI |
| 19 | Reboud J, Xu G, Garrett A, Adriko M, Yang Z, Tukahebwa EM, Rowell C, Cooper JM. Paper-based microfluidics for DNA diagnostics of malaria in low resource underserved rural communities. Proc. Natl. Acad. Sci. U.S.A. 2019;116(11):4834-42. DOI |
| 20 | Ojurongbe O, Adegbosin OO, Taiwo SS, Alli OA, Olowe OA, Ojurongbe TA, Bolaji OS, Adeyeba OA. Assessment of clinical diagnosis, microscopy, rapid diagnostic tests, and polymerase chain reaction in the diagnosis of Plasmodium falciparum in Nigeria. Malar Res Treat. 2013. DOI |
| 21 | Mouatcho JC, Goldring JPD. Malaria rapid diagnostic tests : challenges and prospects. J Med Microbiol. 2013;62(10):1491-505. DOI |
| 22 | Umbers AJ, Unger HW, Rosanas-Urgell A, Wangnapi RA, Kattenberg JH, Jally S, Silim S, Lufele E, Karl S, Ome-Kaius M, Robinson LJ. Accuracy of an HRP-2/panLDH rapid diagnostic test to detect peripheral and placental Plasmodium falciparum infection in Papua New Guinean women with anaemia or suspected malaria. Malar J. 2015;14(412). DOI |
| 23 | Taviad PP, Javdekar TB, Selot BA, Chaudhari VP. Specificity and sensitivity for malaria detection by rapid (parahit) detection test and microscopic method. Natl J Community Med. 2011;2(03):440-2. |
| 24 | Hawkes M, Conroy AL, Opoka RO, Namasopo S, Liles WC, John CC, Kain KC. Use of a three-band HRP2/pLDH combination rapid diagnostic test increases diagnostic specificity for falciparum malaria in Ugandan children. Malar J. 2014;13(43). DOI |
| 25 | Baboo KS, Ndayambaje I, Chizema EK, Silwamba G, Miller J. Effectiveness of rapid diagnosti test for Malaria diagnosis in children under 15 years of age of Nchelenge District in the Luapula Province. Med J Zambia. 2008;35(4).160–5. |
| 26 | Akhtar S, Maimoon S, Wilkinson A, Gowardhan V, Mahore S. Feasible choices in diagnostic methods of malaria. J Vector Borne Dis. 2010;47:151–4. |
| 27 | Ricciardi A, Ndao M. Diagnosis of Parasitic Infections : What’ s Going On ? J Biomol Screen. 2015;20(1):6–21. DOI |
| 28 | Parikh R, Mathai A, Parikh S, Sekhar GC, Thomas R. Understanding and using sensitivity, specificity and predictive values. Indian J Ophthalmol. 2008;56(1):45-51. DOI |
| 29 | Akobeng AK. Understanding diagnostic tests 1 : sensitivity , specificity and predictive values. Found Acta Pædiatrica. 2007;96:338–41. DOI |
| 30 | Bashir M, Sunday E, Mohammed B, Rufa I, Isa H, Sambo KH, et al. Evaluation of the efficacy of rapid diagnostic tests compared to microscopy in the diagnosis of malaria infection. Int J Mosq Res. 2019;6(3):37–41. |
| 31 | Rantala AM, Taylor SM, Trottman PA, Luntamo M, Mbewe B, Maleta K, Kulmala T, Ashorn P, Meshnick SR. Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women. Malar J. 2010;9(269). DOI |
| 32 | Adeola O, Oluyomi S, Sola A, Bassey O, Oluwagbemiga A, Olusola A, Onajole AT, Morakinyo AB. Performance of microscopy method and rapid diagnostic tests in malaria diagnosis amongst pregnant women in Lagos, Southwest Nigeria. Divers Equal Heal Care. 2018;15(3):104–9. DOI |
| 33 | Mbanefo A, Kumar N. Evaluation of Malaria Diagnostic Methods as a Key for Successful Control and Elimination Programs. Trop Med Infect Dis. 2020;3(3). DOI |
| 34 | UNICEF, Centers for Disease Control. Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: round 1 (2008). World Health Organization; 2009. |
| 35 | Adekunle NO, Sam-Wobo SO, Dedeke GA, Ojo D, Abimbola WA, Adeleke MA, Surakat OA. Evaluation of rapid methods in malaria diagnosis from persons attending primary health facilities, Ogun State, Nigeria. Niger J Parasitol. 2014S;35(2):19–25. |
| 36 | Joseph N, Uchila AK. Validation of Malaria Antigen Detecting Rapid Diagnostic Test Kit : A Study from Highly Endemic Area in Coastal India. J Clin Diagnostic Res. 2018;12(9):16–20. DOI |
Cite this article:
Muhammad, I., Abubakar, B., Mohammed, M., Abdullahi, A., Usman, A., Abubakar, S. Determination of malaria rapid diagnostic test effectiveness compared to microscopy (Gold standard). DYSONA – Life Science, 2022;3(2): 49-56. doi: 10.30493/dls.2022.343222
